Abstract
Abstract 4301
Currently, there is no standard reinduction regimen for relapsed and refractory (RR) AML. In order to evaluate responses to reinduction regimens we present a retrospective side-by-side comparison frequently used AML salvage regimens at our institution: CECA, HiDAC and CLAG-M.
From April 2007 to May 2011, 74 consecutive patients with RR AML received CECA (n=44), HiDAC (n=18) or CLAG-M (n=12). The primary outcome was complete remission (CR) rates and secondary outcomes were overall survival (OS) and relapse free survival (RFS). Additional variables such as toxicity and transplant data were also examined.
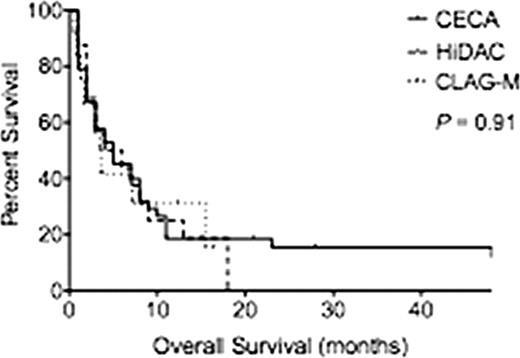
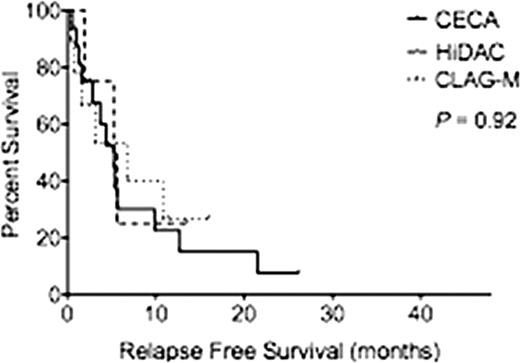
Baseline characteristics among the three groups were similar except for relapse status, with a greater proportion of AML patients receiving CECA after first relapse and HiDAC and CLAG-M after multiple relapses (Table 1). The mean age was 55.6 (range, 24–70), 53.8 (range, 25–71) and 49.5 (range, 23–68) years for CECA, HiDAC and CLAG-M respectively (P=0.38). For CECA, HiDAC and CLAG-M, respectively, 29.5%, 27.7% and 50% of patients were classified with therapy-related AML (P=0.85) and 11.4%, 11.1% and 16.7% of patients were classified with AML transformed from MDS (P=0.62). There was no difference in cytogenetic risk groups between the cohorts with the majority of the patients classified as intermediate risk. The mean blast percentage at the start of treatment was equivalent between the three groups at 39.8%, 27.4% and 26.7% for CECA, HiDAC and CLAG-M respectively (P=0.25). Median follow-up for all patients was 3.5 months. CR rates for CECA, HiDAC and CLAG-M were similar (20.5%, 16.7%, 41.7%; P=0.44). Median OS was 5 months for patients receiving CECA, 5 months for HiDAC and 3.5 months for CLAG-M (P=0.91) (Figure 1A). RFS was also similar among the three groups (p=0.91) (Fig. 1B). Of patients treated with CECA, HiDAC and CLAG-M, 27.2%, 38.8% and 41.6% proceeded to allogeneic hematopoietic cell transplant (P=0.71). Of the 8 patients who achieved CR with CECA but did not proceed to transplant, 5 patients had a CR of less then 3 months, 1 refused further care, 1 was lost to follow up and 1 received further consolidation therapy then relapsed. The mean length of stay in the hospital was 33.6±3.4, 99.7±60.3 and 45.4 ±35.7 days respectively for CECA, HiDAC and CLAG-M (P=0.19). The 30-day treatment related mortality rate was 2.3%, 16.7% and 16.7% respectively (P=0.13). Patients who received CECA had a higher number of total adverse events than patients who received HiDAC and CLAG-M (Table 1), with higher rates of pulmonary edema, sepsis, fungal pneumonia and C. difficle colitis. However, due to the small number of patients per cohort, only descriptive statistics are reported.
(A) Overall Survival by reinduction regimen. (B) Relapsed Free Survival by reinduction regimen.
A.
B.
(A) Overall Survival by reinduction regimen. (B) Relapsed Free Survival by reinduction regimen.
A.
B.
Patient Characteristics, Adverse Events and Causes of Death by Reinduction Regimen.
| Variable . | CECA (n=44) . | HiDAC (n=18) . | CLAG (n=12) . | P value . |
|---|---|---|---|---|
| Mean age, years | 55.6 | 53.83 | 49.7 | 0.38 |
| Gender (n) | 28 | 12 | 4 | 0.13 |
| Male | 16 | 6 | 8 | |
| Female | ||||
| AHD, n (%) | 13 (29.5) | 5 (27.7) | 6 (50) | 0.62 |
| t-AML, n (%) | 5 (11.4) | 3 (11.1) | 2 (16.7) | 0.85 |
| Cytogenetics at diagnosis, n (%) | ||||
| Good risk | 2 (5) | 1 (5.5) | 2 (16.7) | 0.26 |
| Intermediate risk | 17 (38.6) | 10 (55.5) | 6 (50) | |
| Poor risk | 3 (16.6) | 4 (33.3) | ||
| Unknown | 18 (40.9) | 4 (22.2) | 0 (0) | |
| 7 (15.9) | ||||
| Mean blast percentage at start of treatment, % (range) | 39.8 (1–95) | 27.4 (5–75) | 26.69 (7.5–90) | 0.25 |
| Relapse recurrence | ||||
| Relapse 1 | 30 (68.1) | 6 (33.3) | 6 (50) | 0.02 |
| Relapse 2+ | 3 (6.8) | 0 | 2 (16.7) | |
| Primary Refractory | 11 (2.5) | 12 (66.7) | 4 (33.3) | |
| Adverse Events | ||||
| Febrile neutropenia | 10 (22.7) | 4 (22.2) | 2 (16.7) | |
| Pulmonary edema | 2 (4.5) | – | – | |
| Bacteremia | 3 (6.8) | 1 (5.6) | 1 (8.3) | |
| Sepsis | 3 (6.8) | – | – | |
| Fungal pneumonia | 4 (9.1) | – | – | |
| C. Difficile colitis | 2 (4.5) | – | – | |
| 30-Day Toxicity Related Mortality | ||||
| Respiratory failure | – | 1 (5.6) | – | |
| Fungal pneumonia | 1 (2.3) | – | 1 (8.3) | |
| Cardiac arrest | – | 1 (5.6) | 1 (8.3) | |
| Multi-system organ failure | – | 1 (5.6) | – |
| Variable . | CECA (n=44) . | HiDAC (n=18) . | CLAG (n=12) . | P value . |
|---|---|---|---|---|
| Mean age, years | 55.6 | 53.83 | 49.7 | 0.38 |
| Gender (n) | 28 | 12 | 4 | 0.13 |
| Male | 16 | 6 | 8 | |
| Female | ||||
| AHD, n (%) | 13 (29.5) | 5 (27.7) | 6 (50) | 0.62 |
| t-AML, n (%) | 5 (11.4) | 3 (11.1) | 2 (16.7) | 0.85 |
| Cytogenetics at diagnosis, n (%) | ||||
| Good risk | 2 (5) | 1 (5.5) | 2 (16.7) | 0.26 |
| Intermediate risk | 17 (38.6) | 10 (55.5) | 6 (50) | |
| Poor risk | 3 (16.6) | 4 (33.3) | ||
| Unknown | 18 (40.9) | 4 (22.2) | 0 (0) | |
| 7 (15.9) | ||||
| Mean blast percentage at start of treatment, % (range) | 39.8 (1–95) | 27.4 (5–75) | 26.69 (7.5–90) | 0.25 |
| Relapse recurrence | ||||
| Relapse 1 | 30 (68.1) | 6 (33.3) | 6 (50) | 0.02 |
| Relapse 2+ | 3 (6.8) | 0 | 2 (16.7) | |
| Primary Refractory | 11 (2.5) | 12 (66.7) | 4 (33.3) | |
| Adverse Events | ||||
| Febrile neutropenia | 10 (22.7) | 4 (22.2) | 2 (16.7) | |
| Pulmonary edema | 2 (4.5) | – | – | |
| Bacteremia | 3 (6.8) | 1 (5.6) | 1 (8.3) | |
| Sepsis | 3 (6.8) | – | – | |
| Fungal pneumonia | 4 (9.1) | – | – | |
| C. Difficile colitis | 2 (4.5) | – | – | |
| 30-Day Toxicity Related Mortality | ||||
| Respiratory failure | – | 1 (5.6) | – | |
| Fungal pneumonia | 1 (2.3) | – | 1 (8.3) | |
| Cardiac arrest | – | 1 (5.6) | 1 (8.3) | |
| Multi-system organ failure | – | 1 (5.6) | – |
CECA, HiDAC and CLAG-M are equivalent AML salvage regimens in terms of CR, OS, and RFS. Although each resulted in different adverse event profiles. These data provide a basis for designing prospective clinical studies and sample size estimates to formally compare three common reinduction regimens in RR AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal