Abstract
Abstract 4275
There is interest improving induction chemotherapy in acute myeloid leukemia. The PALG (Polish Adult Leukemia Group) demonstrated that the addition of cladribine to daunorubicin/cytarabine resulted in improved CR rates and overall survival in untreated AML patients <60 years of age (Blood{ASH Annual Meeting Abstracts}, Nov 2009; 114: 2055). Idarubicin has shown superiority over high dose daunorubicin in the Acute Leukemia French Association 9801 Study (J Clin Oncol 2010, 28: 808–814), and here we present results of idarubicin/cytarabine/cladribine (IAC) therapy in untreated AML.
Records of all patients receiving cladribine at Saint Louis University Hospital between December 2008 and July 2011 were analyzed after identification using a pharmacy database search. We began using cladribine for all acute myeloid leukemia patients after the report of improved overall survival in December 2008 (Blood{ASH Annual Meeting Abstracts}, Nov 2008; 112: 133). All patients with untreated acute myeloid leukemia by World Health Organization (WHO) criteria were included who received induction with IAC, which consisted of: idarubicin 12mg/m2 intravenously (IV) daily day 1–3, cytarabine 200mg/m2 IV daily day 1–7 by continuous infusion, and cladribine 5mg/m2 IV daily day 1–5.
34 patients received the IAC regimen. International Working Group criteria were used to assess response and cytogenetic abnormalities were grouped according to SWOG criteria. Median age was 60 years (range 25–81). Age: < 60: 18/34 (53 %), ≥ 60: 16/34 (47 %). 15/34 (44 %) had ECOG performance status 1–2, and 19/34 (56 %) had performance status 3–4. Median WBC count on presentation was 11 K (range 1K–483K). Risk distribution by cytogenetics: favorable: 4/34 (12 %), intermediate: 20/34 (59 %), adverse: 10/34 (29%) and by molecular analysis with NPM1/FLT3-ITD/CEBPA: favorable: 7/34 (21 %), intermediate-1: 12/34 (35 %), intermediate-2: 5/34 (15 %), adverse: 10/34 (29 %).
The ORR (CR+CRi): 22/34 (65 %). CR 21, Cri 1. ORR by age: < 60: 14/18 (78 %), ≥ 60: 8/16 (50 %). ORR by cytogenetics: favorable: 4/4 (100%), intermediate: 14/20 (70 %), adverse: 4/10 (40 %). CR/CRi in 1 course: 18/20 (90%). Treatment failure (TF): 12 (37%). Treatment failure was due to persistent leukemia in 6/12 (50 %), death in bone marrow aplasia in 5/12 (42 %), and death due to indeterminate cause in 2/12 (8 %).
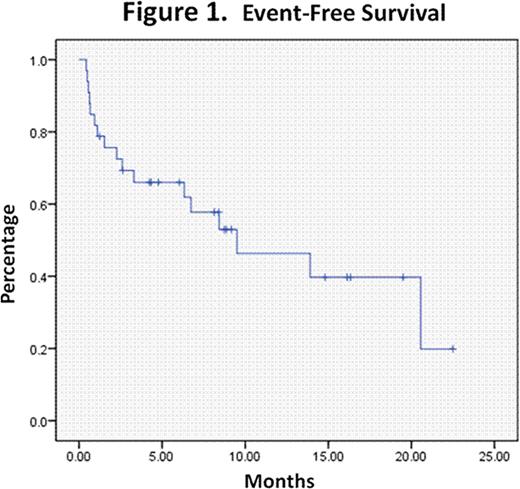
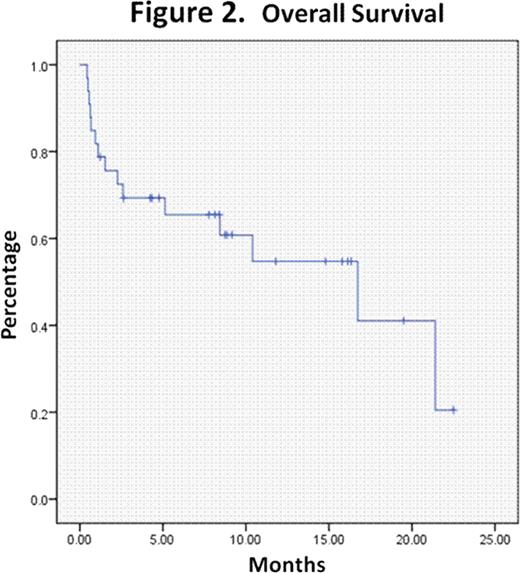
Further consolidation treatment was heterogenous and typically consisted of either HIDAC or allogeneic transplantation depending on risk features. The median observation time of this patient cohort was 16.7 months (range: 4.1–29.4 months). The median event-free survival and overall survival were 9.5 months (range: 1.4–17.6 months) and 16.7 months (range: 5.1–28.3), respectively. See Figures1 and2.
The 30 and 60 day mortality rates were 3/34 (9 %) and 7/34 (21 %), respectively. The median time to hospital discharge in those achieving remission was 26.5 days (range: 21–80 days). The median day to achievement of a neutrophil count >500 was 23 (range 21–43) in those who achieved a CR in 1 course. The median day to achievement of platelet transfusion independence with transfusion threshold of 10K and to platelet count ≥ 50K was 21 (range 19–28) and 23 (range 20–26), respectively, in those who achieved a CR in 1 course. The median number of red blood cell and platelet transfusions in those who achieved CR in 1 course was 8 (range 2–18) and 6 (range 3–18), respectively. Death in aplasia etiologies were (all in 1st IAC induction unless otherwise noted): autopsy-proven Aspergillus infections, one disseminated day 14, one pulmonary day 16/ MDR Pseudomonas sepsis and toxic megacolon day 21/ respiratory failure of undetermined etiology with recent stroke day 16 of 2nd IAC induction. Indeterminate deaths were: shock of undetermined etiology with multiorgan failure day 16 / probable Zygomycete pulmonary infection day 37. 25/34 patients (74%) had documented infection during induction.
We found a high complete remission rate of IAC in younger patients with an acceptable 30 day mortality rate in spite of short follow-up. We believe that the addition of cladribine to induction chemotherapy with intensified daunorubicin or idarubicin for acute myeloid leukemia warrants further study.
Off Label Use: Cladribine was utilized off-label in this study based on prior reports of efficacy in untreated and relapsed acute myeloid leukemia patients.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal