Abstract 4248
According to US SEER data, 10.3% of patients diagnosed with Acute Lymphoblastic Leukemia (ALL) during 2004–2008 were 20–34 years old. The majority of patients, 60.3%, diagnosed were under 20, and the remainder were over 35 years of age. Many retrospective trials have looked at treatment of adolescents and young adults (AYA) with ALL, and overall survival (OS) rates after treatment with pediatric regimens versus adult protocols. European studies done in the early 2000’s found improvement in OS and progression free survival (PFS) in this population when treated with pediatric regimens. Differences in pediatric versus adult regimens include increased doses of non-myelosuppressive drugs such as glucocorticoids, Vincristine and Aspariginase, more frequent CNS prophylaxis, and longer maintenance therapy. These differences, unfortunately, often cause harsh toxicities, not well tolerated by adults. Here we present our experience at Moffitt Cancer Center (MCC), in treating AYA’s (age <30 years) with ALL after treatment with pediatric or adult protocols.
We used the MCC Total Cancer Care database to identify patients with ALL who underwent intensive therapy. Patient medical records were reviewed, and patients between 18–30 years of age were included in this analysis. Hyper-CVAD was the most common adult regimen, while CALGB 10403 and ECOG 2993 were the most common pediatric regimens. Descriptive data were reported, t-test was used to compare continuous variables while chi square test was used for categorical variables. Kaplan Meier curves were used for OS and PFS. Log rank test was used to compare survival times between groups. Cox regression was used for multivariable analysis. All analyses were conducted using SPSS version 19.0.
Between 1/1/2004 and 6/30/2011, 42 AYA ALL patients were treated at MCC. Males accounted for 74%, while 26% were female. Hyper-CVAD was used as frontline chemotherapy in 64% of patients, while 31% received a pediatric regimen. Of the 42 patients, 64% were white, 5% African American, 12% Hispanic and 19% were classified as other. Pre-B ALL made up 69% of patients, while 31% had T-cell origin. Cytogenetics category at diagnosis was adverse in 29% of patients, intermediate in 55% and favorable in 12% of the population, while 5% were unknown. Philadelphia chromosome was positive in 17%. At diagnosis 38% had lymphadenopathy, 33% had splenomegaly and 12% had CNS disease. Overall, 45% underwent allogeneic stem cell transplant (SCT) in CR1, while 55% did not.
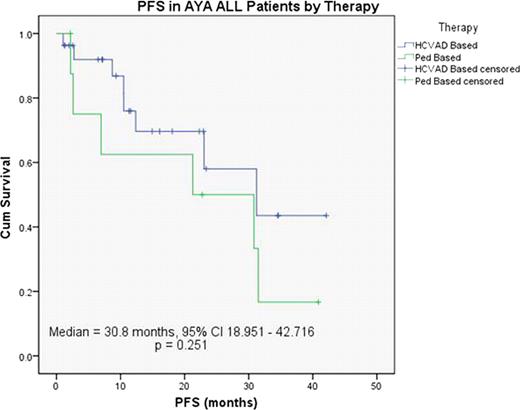
Overall, 85% of patients achieved CR, including 81% receiving Hyper-CVAD and 92% with pediatric regimens. Median PFS was 30.8 months (95% CI 21.9–39.7) for the entire AYA population, 31.2 months (95% CI 13.2–49.3) in the Hyper-CVAD group and 21.3 months (95% CI 0.0–49.6) in the pediatric regimen group. (P=0.124) (Figure 1)
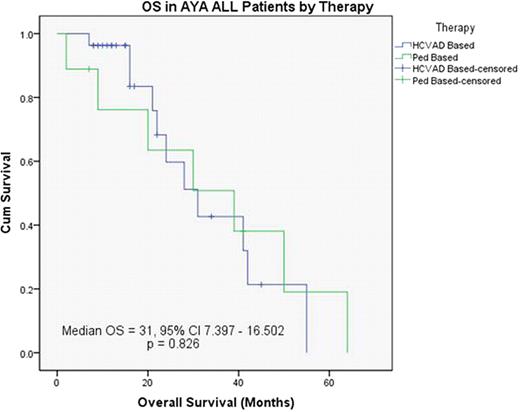
Median OS in the entire AYA population was 31 months (95% CI 15.3–46.7). OS with Hyper-CVAD was 31 months (95% CI 19.7–42.2) versus 30 months (95% CI 3.1–56.9) in the pediatric group (P=0.984). (Figure 2)
Median PFS was 44.8 months in patients who had an allogeneic SCT versus 23 months in those who did not (P=0.009). Further, there was a trend toward worse outcomes in patients with adverse cytogenetics.
Median OS in patients who had an allogeneic SCT was 39 months (95% CI 16.8–61.2) versus 31 months (95% CI 15.2–46.8) in those who did not. (P=0.424).
In univariable analysis, no difference in outcome was found based on race, gender, T or B cell origin, lymphadenopathy or splenomegaly. Using Cox regression analysis, Philadelphia chromosome positivity (P= 0.009), elevated WBC (P=0.008) and CNS disease at diagnosis (P=0.029) were statistically significant independent variables for OS for all patients.
Optimal treatment of AYA with ALL remains unclear. The role of pediatric versus adult regimens remains controversial, and further prospective investigation is necessary. In our experience, outcomes in AYA appear similar between Hyper-CVAD and pediatric regimens. Improvement in PFS is noted if patients are able to undergo allogeneic SCT. In addition, Philadelphia chromosome positivity, elevated WBC at diagnosis and CNS disease appear to be independent variables that negatively affect prognosis in these young patients, for whom more novel therapies are needed.
No relevant conflicts of interest to declare.
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal