Abstract
Abstract 4242
Acute lymphoblastic leukemia (ALL) and acute myelogenous leukemia (AML) account for approximately 26% of all pediatric cancers. Anthracyclines are critical in the treatment of ALL and AML; however, their use is limited by cardiotoxicity. Dexrazoxane (DZX) is an FDA-approved cardioprotective agent that chelates intracellular free iron and iron bound to anthracyclines. Evidence for the efficacy of DZX as a cardioprotectant in children is limited, but available data support a short term cardioprotective effect in patients with ALL. The frequency of DZX use in pediatric cancer patients at high risk of developing cardiac toxicity is unknown.
We sought to describe patterns of DZX use between 1999 and 2009 in a retrospective cohort study of pediatric patients diagnosed with ALL or AML in 43 pediatric hospitals across the United States in the Pediatric Health Information Systems (PHIS) database.
Patients previously identified and validated as having de novo ALL and AML at these PHIS hospitals were included. Demographic data, as well as chemotherapy and DZX use were obtained from PHIS.
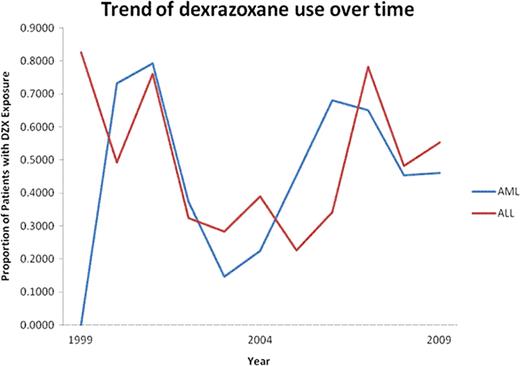
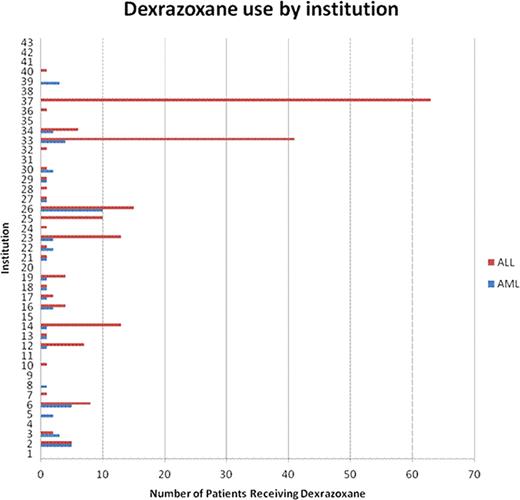
Of 8733 patients with de novo ALL and 2556 with de novo AML, 207 and 52 received DZX, respectively. The proportion of patients receiving DZX was higher in older children with ALL or AML and in African-Americans and males with ALL (Table 1). There was no trend in the proportion of patients receiving DZX per year in either group (Figure 1). DZX use varied by region in ALL, with 55% of the patients receiving DZX being treated in the northeast, but was not significantly different in AML. There was substantial variability in prescribing practices across institutions (Figure 2). Most patients with ALL and AML received the first dose of DZX in the first 30 days (67% and 31%, respectively) or >360 days from their first leukemia admission (23.5% and 31%, respectively).
Demographic characteristics of the patients in the PHIS ALL and AML cohorts
| . | ALL . | P . | AML . | P . | ||
|---|---|---|---|---|---|---|
| ALL patients w/o DZX exp (n=8526) . | ALL patients w/DZX exp (n=207) . | AML patients w/o DZX exp (n=2504) . | AML patients w/DZX exp (n=52) . | |||
| Age, n (%) | <.001 | .042 | ||||
| <1 year | 252 (3) | 0 (0) | 254 (10) | 1 (2) | ||
| 1 to <5 years | 3777 (44) | 47 (23) | 623 (25) | 14 (27) | ||
| 5 to <10 years | 2211 (26) | 45 (22) | 426 (17) | 4 (7) | ||
| 10 to <15 years | 1409 (17) | 70 (34) | 567 (23) | 12 (30) | ||
| 15 to <20 years | 826 (10) | 41 (20) | 574 (23) | 18 (27) | ||
| ≥ 20 years | 51 (0.6) | 4 (2) | 60 (2) | 3 (4) | ||
| Sex, n (%) | .038 | .600 | ||||
| Male | 4777 (56) | 131 (63) | 1353 (54) | 30 (58) | ||
| Female | 3749 (44) | 76 (37) | 1151 (46) | 22 (42) | ||
| Race, n (%) | .024 | .595 | ||||
| White | 6395 (75) | 155 (75) | 1707 (68) | 40 (77) | ||
| Black | 616 (7) | 27 (13) | 309 (12) | 4 (9) | ||
| Asian | 252 (3) | 6 (3) | 85 (3) | 1 (2) | ||
| Native American | 42 (0.5) | 2 (1) | 14 (0.5) | 0 (0) | ||
| Other | 586 (7) | 13 (6) | 182 (7) | 5 (9) | ||
| Missing | 631 (7) | 8 (4) | 207 (8) | 2 (4) | ||
| Ethnicity, n (%) | .042 | .029 | ||||
| Hispanic | 1952 (23) | 47 (23) | 472 (19) | 17 (34) | ||
| Non-Hispanic | 87 (1) | 7 (3) | 51 (2) | 0 (0) | ||
| Unknown | 6485 (76) | 155 (74) | 1981 (79) | 35 (66) | ||
| Missing | 0 (0) | 0 (0) | 0 (0) | |||
| Region, n (%) | <.001 | .002 | ||||
| Midwest | 2328 (27) | 19 (0.5) | 724 (29) | 10 (19) | ||
| Northeast | 715 (8) | 113 (55) | 250 (10) | 13 (25) | ||
| South | 2876 (34) | 38 (18) | 870 (35) | 13 (25) | ||
| West | 2607 (31) | 37 (18) | 660 (26) | 16 (31) | ||
| Anthracycline | ||||||
| Doxorubicin | 171 (82) | 1 (2) | ||||
| Daunorubicin | 15 (7) | 16 (31) | ||||
| Mitoxantrone | 2 (1) | 28 (54) | ||||
| Idarubicin | 3 (1) | 3 (6) | ||||
| Unknown | 17 (8) | 4 (8) | ||||
| . | ALL . | P . | AML . | P . | ||
|---|---|---|---|---|---|---|
| ALL patients w/o DZX exp (n=8526) . | ALL patients w/DZX exp (n=207) . | AML patients w/o DZX exp (n=2504) . | AML patients w/DZX exp (n=52) . | |||
| Age, n (%) | <.001 | .042 | ||||
| <1 year | 252 (3) | 0 (0) | 254 (10) | 1 (2) | ||
| 1 to <5 years | 3777 (44) | 47 (23) | 623 (25) | 14 (27) | ||
| 5 to <10 years | 2211 (26) | 45 (22) | 426 (17) | 4 (7) | ||
| 10 to <15 years | 1409 (17) | 70 (34) | 567 (23) | 12 (30) | ||
| 15 to <20 years | 826 (10) | 41 (20) | 574 (23) | 18 (27) | ||
| ≥ 20 years | 51 (0.6) | 4 (2) | 60 (2) | 3 (4) | ||
| Sex, n (%) | .038 | .600 | ||||
| Male | 4777 (56) | 131 (63) | 1353 (54) | 30 (58) | ||
| Female | 3749 (44) | 76 (37) | 1151 (46) | 22 (42) | ||
| Race, n (%) | .024 | .595 | ||||
| White | 6395 (75) | 155 (75) | 1707 (68) | 40 (77) | ||
| Black | 616 (7) | 27 (13) | 309 (12) | 4 (9) | ||
| Asian | 252 (3) | 6 (3) | 85 (3) | 1 (2) | ||
| Native American | 42 (0.5) | 2 (1) | 14 (0.5) | 0 (0) | ||
| Other | 586 (7) | 13 (6) | 182 (7) | 5 (9) | ||
| Missing | 631 (7) | 8 (4) | 207 (8) | 2 (4) | ||
| Ethnicity, n (%) | .042 | .029 | ||||
| Hispanic | 1952 (23) | 47 (23) | 472 (19) | 17 (34) | ||
| Non-Hispanic | 87 (1) | 7 (3) | 51 (2) | 0 (0) | ||
| Unknown | 6485 (76) | 155 (74) | 1981 (79) | 35 (66) | ||
| Missing | 0 (0) | 0 (0) | 0 (0) | |||
| Region, n (%) | <.001 | .002 | ||||
| Midwest | 2328 (27) | 19 (0.5) | 724 (29) | 10 (19) | ||
| Northeast | 715 (8) | 113 (55) | 250 (10) | 13 (25) | ||
| South | 2876 (34) | 38 (18) | 870 (35) | 13 (25) | ||
| West | 2607 (31) | 37 (18) | 660 (26) | 16 (31) | ||
| Anthracycline | ||||||
| Doxorubicin | 171 (82) | 1 (2) | ||||
| Daunorubicin | 15 (7) | 16 (31) | ||||
| Mitoxantrone | 2 (1) | 28 (54) | ||||
| Idarubicin | 3 (1) | 3 (6) | ||||
| Unknown | 17 (8) | 4 (8) | ||||
To our knowledge, this is the only description of DZX use in patients with ALL and AML using national, population-based data. Older children and those treated in the Northeastern U.S. were more likely to have received DZX, with the first dose being given either early or late in the study period. In general, DZX administration was relatively rare and was driven by only a few institutions, especially in ALL patients. This lack of universal acceptance of DZX is likely multi-factorial, including concerns about acute and long-term side effects, impact on the antitumor effect of anthracyclines, and risk for secondary malignant neoplasm. Work is ongoing to substantiate or refute these concerns using PHIS data.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal