Abstract
Abstract 4216
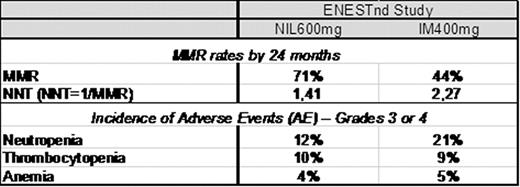
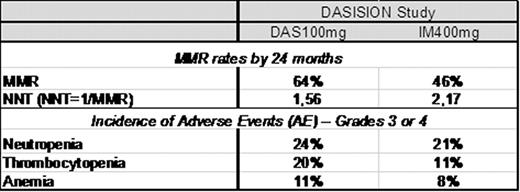
The use of imatinib (IM) has greatly improved the outcome of patients with Ph+ chronic myeloid leukemia (CML), with a good balance of potency and exposure that translated into clinical efficacy for many patients. However, patient responses to IM can be variable with factors such as blood plasma levels and OCT-1 transporter activity influencing responses (Larson, 2008; White, 2010). Some patients do not reach complete cytogenetic response (CCyR) and others may have intolerable side effects. Loss of response to IM and disease progression occur mainly in the first 3 years of treatment, and the rate of overall survival is affected as a consequence. Molecular monitoring is the most sensitive measure of CML disease burden and Major Molecular Response (MMR) is associated with an extremely low rate of disease progression (Hughes, 2008). Objectives: (1) To estimate the ‘Number Needed to Treat' (NNT) to achieve one MMR by 24 months. (2) To provide a relative measure of the cost of attaining treatment objective defined by MMR and hematologic adverse events (AEs) with 2 novel targeted therapies in the treatment of newly diagnosed CP-CML from the Brazilian Public Health Care System (SUS) perspective. Methods: MMR and AE data were evaluated from two phase 3 studies comparing IM 400mg once daily with DAS 100mg (Hochhaus, Haematologica. 2011; abstract 1011) and the other with NIL 300mg twice daily (Hochhaus, Haematologica. 2011, abstract 484) in newly diagnosed CP-CML patients. MMR rates by 24 months were used to calculate the NNT as the inverse of the MMR rate. The costs of managing neutropenia (R$8137), thrombocytopenia (R$599) and anemia (R$232) were estimated from SUS perspective in local currency (2008 BRL) using administrative databases, literature and survey with clinical experts. The costs of managing the AEs were then multiplied by the incidence of the AEs. Results: The number of newly diagnosed Ph+ CML-CP patients needed to treat to achieve 1 MMR with nilotinib (NNT=1,41) is 38% lower than imatinib in the ENESTnd study (Table 1). The dasatinib NNT of 1,56 is 28% lower than imatinib in the DASISION study (table 2). Lower NNT represents more effective therapy. The NNT analysis highlights that CML treatment with NIL600mg or DAS100mg provides greater efficacy when each are compared with IM400mg in the studies, and that Nilotinib NNT of 1,41 in the ENESTnd study is the most efficient in producing MMR among BCR-ABL inhibitors. DASISION study results demonstrate dasatinib produced more hematologic events, resulting in an estimated 14% higher cost of managing these adverse events compared to imatinib (table 2). ENESTnd study results demonstrate nilotinib produced lower incidence of hematologic events than imatinib, providing savings around 40% lower than imatinib in hematologic AE grade 3 or 4 management (table1). Conclusion: Although comparisons between published studies have some limitations, such an approach may be useful in the absence of direct comparative data. The NNT findings in this 24-months analysis and the differential cost of managing AEs in each treatment from this evaluation suggests that nilotinib provides better clinical outcomes and would result in lower costs for hematologic AE management from the perspective of the Brazilian Public Health Care System when compared to Imatinib.
MMR rates and incidence of adverse events reported in ENESTnd study – 24 months
MMR rates and incidence of adverse events reported in DASISION study – 24 months
Alves:Novartis: Employment. Coombs:Novartis: Employment. Ruiz:Novartis: Employment. Valentim:Novartis: Employment. Lemos:Novartis: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal