Abstract
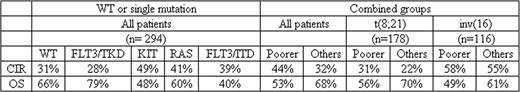
Patients with core binding factor (CBF) AML [t(8;21) and inv(16)/t(16;16)] are generally excluded from transplantation in first CR because of their relatively favorable prognostic outcome with conventional chemotherapy. CBF AML is heterogeneous, however, with a number of molecular mutations described that may influence outcome. To ascertain whether the impact of gene mutations known to be recurrent in CBF AML is sufficient to justify alternative therapy such as transplantation in first CR, we have carried out mutational analysis of 355 young adult CBF AML patients treated on the UK MRC/NCRI AML10, 12 and 15 trials between 1988 and 2009. Median age was 40 years, 95% were <60 years. 200 had t(8;21) and 155 inv(16), overall survival (OS) at 5 years 65% and 56% respectively. In total, 28% had a KIT mutation (exons 8 [extracellular], 10+11 [transmembrane] and 17 [tyrosine kinase domain]), 26% an N- and/or K-RAS mutation (codons 12, 13 and 61), 6% a FLT3/ITD, 10% a FLT3/TKD mutation and 6% a c-CBL mutation (exons 8 and 9). Of the 100 KIT-MUT patients, 54% were only in exon 17, 28% in exon 8, 8% in exon 10+11, and 10% had mutations in 2 domains. KIT mutations were not associated with age or disease type (de novo/secondary) but were higher in males than females (33% with vs 22% without a mutation, P=.03) and were associated with a higher WCC (median 28.7×109/L vs 14.7×109/L, P=.01). Of the 94 RAS-MUT patients, 65% were in N-RAS, 32% K-RAS and 3% both. RAS mutations were not associated with age, disease type or sex, but were associated with a higher WCC (median 27.0 ×109/L vs 16.7×109/L, P=.03). There was a negative correlation between KIT and RAS mutations (P<.0001). Excluding patients with a mutation in more than one gene or at more than one location in a specific gene, 294 patients were either wild-type (WT) for all 5 genes (39%) or had a mutation just in KIT (22%), FLT3/ITD (5%), FLT3/TKD (6%), RAS (24%) or CBL (4%). Overall CR rate was high (94%) and no mutation had a significant impact on this. The cumulative incidence of relapse (CIR) and OS were analyzed for each mutational category as both parameters might influence the decision to change therapy. CIR at 5 years was lower and OS higher in the WT and FLT3/TKD-MUT patients than in the poorer prognosis KIT-MUT, RAS-MUT, FLT3/ITD-MUT patients (P=.05 and.01 respectively across all groups) (see Table). If the 3 poorer prognostic categories were combined together, CIR and OS were significantly different compared to the remaining patients (P=.002 and.005 respectively). Comparing t(8;21) and inv(16), KIT mutations were more frequent in inv(16) than t(8;21) (34% vs 23%, P=.01), particularly exon 8 mutations (20% vs 3%, P<.0001), as were RAS (37% vs 18%, P<.0001) and FLT3/TKD mutations (15% vs 6%, P=.006), whereas FLT3/ITDs were more frequent in t(8;21) (3% vs 9%, P=.04). There was no significant heterogeneity between t(8;21) and inv(16) in the impact on OS of the combined group (KIT-MUT, FLT3/ITD, RAS-MUT). In both subtypes the poorer prognostic group did worse than the others (P=.06 for t(8;21), P=.08 for inv(16)) (Table 1). However, for CIR, the poorer risk mutational status had an apparent impact in t(8;21) (P=.09), but not inv(16) patients (P=.2). This implies that in t(8;21) the impact of gene mutations is predominantly seen in the long-term response to first line therapy whereas in inv(16), the good overall survival is in large part due to the excellent response to salvage therapy, with the presence of gene mutations predominantly influencing the response to therapy after relapse has occurred. No cases of t(8;21) were identified with sufficient relapse risk to justify the risks of allogeneic transplantation in first remission, but it may be appropriate to consider intensification in inv(16) with either a KIT or RAS mutation or FLT3/ITD.
Disclosures:
No relevant conflicts of interest to declare.

This icon denotes a clinically relevant abstract
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal