Abstract
Abstract 4171
Hematopoietic stem cell (HSC)-targeted gene therapy approaches utilizing HIV1-based lentiviral vectors is a promising modality for a number of inherited and acquired disorders affecting the blood. Unlike cell lines and murine HSCs, human HSCs demonstrate lower transduction efficiency with HIV1 vectors, potentially limiting this approach. Lentiviral transduction requires four essential steps: (1) internalization, (2) reverse transcription, (3) nuclear transport, and (4) integration into genomic DNA. In this study, we sought to evaluate which step limits transduction of human CD34+ cells with HIV1 vectors.
We transduced HeLa cells and human CD34+ cells with a self-inactivating-HIV1 vector at low and a 10-fold-higher multiplicity of infection (MOI) (MOI 0.5 vs. MOI 5 for HeLa cells and MOI 5 vs. MOI 50 in CD34+ cells, respectively) and assayed each of the four steps based upon the rationale that if a given step was not rate limiting, a 10-fold-greater value would be obtained at the 10-fold-higher MOI. Amounts of the vectors were determined by real time PCR using RNA and DNA samples extracted from the transduced cells over time. The ratios of vector genome amounts at high MOI to amounts at low MOI were compared between CD34+ cells and HeLa cells to determine whether there was a limiting step for CD34+ cell transduction.
To evaluate RNA internalization, we determined relative amounts of vector RNA in the cells at 5 min, 10 min, 30 min, and 60 min after transduction. Among HeLa cells, relative amounts of vector RNA showed a rapid increase and then plateaued at 30 min after transduction, while in the CD34+ cells, relative amounts of vector RNA increased gradually over 60 min. When we calculated the ratios of vector RNA amounts at high MOI to low MOI for each type of cell, we observed similar ratios when comparing HeLa to CD34+ cells at 60 min after transduction. These data suggest that despite slower internalization of vector RNA in CD34+ cells, it is not a limiting step in lentiviral transduction.
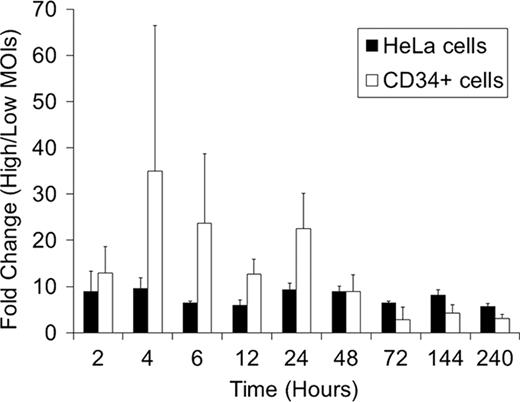
To evaluate reverse transcription, nuclear transport, and integration; we determined relative amounts of vector DNA at 2 hrs, 4 hrs, 6 hrs, 12 hrs, 24 hrs, 2 days, 3 days, 6 days, and 10 days after transduction. We detected similar patterns of relative vector DNA in both Hela and CD34+ cells: as their relative amounts increased, they reached a peak at 24 hrs (reverse transcription), decreased, and then reached a plateau at 2–3 days after transduction (nuclear transport and integration). CD34+ cells showed higher ratios of vector DNA at high MOI to low MOI at 2–24 hrs, similar ratios at 2 days, and lower ratios at 3–10 days, compared to HeLa cells (Figure). These data suggest that transduction of human CD34+ cells has a limitation around 2 days after vector exposure corresponding to nuclear transport or integration.
To evaluate nuclear transport for both types of cells, we compared the relative amounts of vector DNA to total DNA and nuclear DNA. Nuclear DNA was extracted from the nuclear fractions of transduced cells which had been isolated by a sucrose gradient. In both cells, the nuclear DNA had tendencies to show lower amounts of vector DNA at 6–48 hrs after transduction and similar or higher amounts of vector DNA at 2–7 days, compared to the total DNA. These data suggest that vector DNA is transported to the nucleus in about 2–3 days.
Taken together, these data suggest that nuclear transport is a limiting step in lentiviral transduction of human CD34+ cells. We hypothesized that transduction efficiency for human CD34+ cells might increase under conditions that induce greater cell expansion, since dividing cells do not have intact nuclear membranes. We compared relative amounts of vector DNA in human CD34+ cells transduced in X-VIVO10 medium (standard) and StemlineII medium (greater expansion) at 12 hrs, 24 hrs, 2 days, 3 days, 5 days, and 7 days after transduction. StemlineII exposed cells showed higher amounts of vector DNA, compared to X-VIVO10, at all time points except 24 hrs. The data suggest that StemlineII increases transduction efficiency for human CD34+ cells by increasing nuclear transport.
In summary, we demonstrate that nuclear transport is a limiting step in lentiviral transduction of human CD34+ cells and that StemlineII medium could increase transduction efficiency. These data are helpful for the design of strategies to improve upon lentiviral transduction for human CD34+ cells by improving nuclear transport.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal