Abstract
Abstract 4062
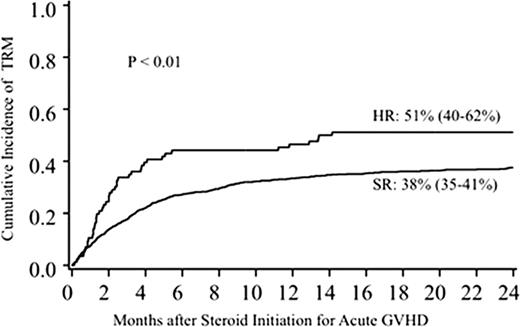
To define high risk acute graft-versus-host disease (GVHD) at its onset, we examined the initial GVHD stage and grade of 864 patients at the University of Minnesota who received uniform therapy with prednisone 60 mg/m2/d. We compared the prognostic utility of the Minnesota (MN) (modified from Consensus) versus Center for International Blood and Marrow Transplant Research (CIBMTR) GVHD organ stage-derived grading systems. As neither GVHD grading system optimally predicted outcomes, a novel acute GVHD risk score was devised by combining the MN and CIBMTR systems. Using multiple regression analysis, we could dichotomize patients into (high risk, HR, n=86) acute GVHD with initial grade IIIC, IID or IVD who were significantly less likely to respond to steroid therapy by day 28 than patients with standard risk (SR, initial grade IA-IIIB, n=778) GVHD (Univariate complete response [CR]+ partial response [PR] at day 28: HR 43% [95% CI 33–53%] vs. SR 68% [95% CI 65–71%], p <.001; Multivariate HR Relative Risk [RR] of overall response [CR+PR], 0.3, 95% CI, 0.2–0.5, p<.001). The probability of treatment-related mortality (TRM) at 2 years was significantly higher in patients with HR than SR acute GVHD (51% [95% CI 40–62%] vs. 38% [95% CI 35–41%], p <.01) (figure).
In multivariate analysis, patients with HR acute GVHD had a 2 fold increase risk of TRM (RR, 2.0, 95% CI, 1.4–2.8, p<.001) and similarly higher 2 year mortality (RR, 1.5, 95% CI, 1.1–2.1, p=.004). Using this novel acute GVHD Risk Score, we can identify initially HR GVHD as either skin stage 4, lower gastrointestinal (GI) stage 3+, liver stage 3+, or skin stage 3 and lower GI or liver stage 2+ GVHD. Patients with HR acute GVHD at onset have a poor prognosis, require alternative initial therapy and should be the focus of novel therapeutic trials.
Disclosures:
Weisdorf:Genzyme: Consultancy, Research Funding.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal