Abstract
Abstract 4006
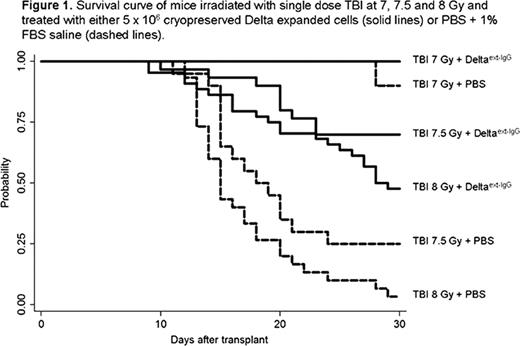
Despite the large body of research designed to assess the effects of radiation on hematopoiesis, specific therapies aimed at improving the survival of individuals accidentally exposed to higher doses of whole body irradiation are lacking. The ideal therapeutic agent would be an immediately available, easily distributable single agent therapy able to generate at least temporary in vivo hematopoietic recovery, especially of the myeloid lineage, and facilitate return of autologous hematopoiesis. We have previously demonstrated that murine lin−/sca-1+/c-kit+ (LSK) cells which have been ex vivo expanded in the presence of an immobilized Notch ligand form consisting of the extracellular domain of Delta1 fused to the Fc domain of human IgG1 (Delta1ext-IgG) can engraft across MHC barriers. We now show that the same product is also capable of reducing radiation toxicity and improving overall survival. Methods: LSK cells were isolated by flow cytometry from whole bone marrow (BM) of Ly5a mice (H-2b, CD45.1) and placed in culture in the presence of immobilized Notch ligand and Iscove's medium supplemented with cytokines mSCF, hFlt-3 ligand, hIL-6, and hIL-11. After 14 days, expanded cells were harvested and cryopreserved in 90% FBS + 10% DMSO. On the day of transplant, frozen cells were thawed, washed, and resuspended in PBS + 1% FBS. To determine the ability of the expanded cells to contribute to in vivo hematopoietic recovery and provide radioprotection in a MHC mismatched model, Balb-c recipient mice (H-2d, CD45.2) were treated with single dose TBI at 7, 7.5 or 8 Gy followed by infusion of either a PBS + 1% FBS solution (PBS) or 5 × 106expanded cells. The results presented herein represent the combined data from 3 consecutive experiments, including a total of 150 mice for the generation of survival curves and an additional 54 mice to assess donor engraftment. All experiments were approved by the Animal Care and Use Committee (IACUC), animals were observed for a minimum of 30 days to assess survival. Moribund animals that met specific criteria established by the IACUC were euthanized and counted as an experimental death. Separately, mice were sacrificed at 1, 2, and 3 weeks after infusion and peripheral blood and BM collected to determine the degree of donor hematopoiesis by flow cytometry. PB was also collected at day 45 to evaluate donor chimerism and total white counts (WBC) recovery. Results: At a TBI dose of 8 Gy, 97% of mice that received PBS died in contrast to only 52% mortality in the group treated with 5 × 106expanded cells (p<0.0001). Similarly, at a dose of 7.5 Gy, the survival rate at 30 days was 25% and 70% for mice that received PBS and expanded cells, respectively (p=0.0004). When the TBI doses was reduced to 7 Gy, all mice infused with expanded cells survived and only one died in the PBS group (p=0.22) (Figure 1). Of note, no further deaths were observed after 30 days and mice that received 8 Gy recovered WBC by day 45. Evaluation of donor derived hematopoiesis demonstrated in vivo persistence of the mismatched expanded cells through day 28, with peak engraftment occurring before day 14. The percent of donor cells in the peripheral blood one week following infusion ranged between 50 to 95% in all mice that received expanded cells, with higher donor engraftment correlating with increased dose of TBI. The level of donor engraftment then decreased over time, but in the surviving mice remained persistent to levels ranging from 10 to 30%. Importantly, no signs of graft versus host disease (GVHD) were observed despite the full-mismatch between the recipient and donor product. Conclusions: Ex vivo expanded and cryopreserved murine hematopoietic progenitors can be infused safely and effectively to provide temporary donor derived hematopoiesis and rescue mice after lethal acute ionizing radiation. Importantly, such cells can be ex vivo expanded, cryopreserved, and banked for future treatment of neutropenia as a result of accidental radiological exposures or therapies. Furthermore, in vivo transplantation studies demonstrate that short-term engraftment without manifestations of GVHD can be attained across major H-2 barriers, supporting the use of this product as a universal or third party donor product. Studies are on-going aimed at determining an optimal range of cell doses and extending the window after radiation exposure when the cryopreserved, expanded products can be effective.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal