Abstract
CD138 expression is a hallmark of plasma cells. However, decreased expression of CD138 is frequently found although its clinical significance is unknown. To evaluate the significance of decreased CD138 expression, we examined clinical phenotypes of cases with decreased CD138 expression (CD138-dim cases). Furthermore, we attempted to elucidate the mechanism(s) regulating CD138 expression by utilizing two MM cell lines, KYMM-1 and KYMM-2, established from a CD138-dim case.
CD138 expression levels were determined in 107 primary MM cases using flowcytomerty with the CD38 gating method. Cases with more than 20% of MM cells with CD138 negative population were categorized as CD138-dim cases. Real time RT-PCR was utilized to analyze gene expression. Two MM cell lines, KYMM-1 and KYMM-2, were established from pleural effusion of a CD138-dim case. Both KYMM-1 and KYMM-2 had high CD38 expression with cytoplasmic kappa light chains, while lacking CD19 and CD20 expression. EB virus infection was undetectable with PCR in both cell lines. Identical DNA fingerprinting profile (Cell ID™ System, Promega KK, JAPAN) was observed in patient's primary pleural effusion cells, KYMM-1 and KYMM-2. We also analyzed methylation status of the promoter region of CD138, IRF4 and Blimp-1 by treating KYMM-1 genomic DNA with sodium bisulfite.
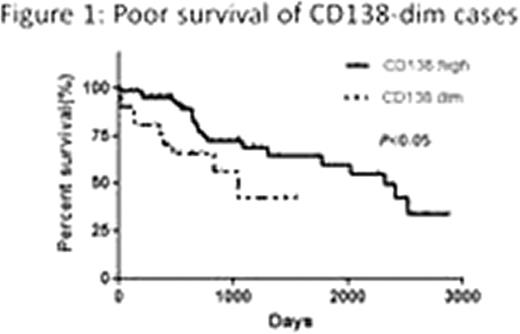
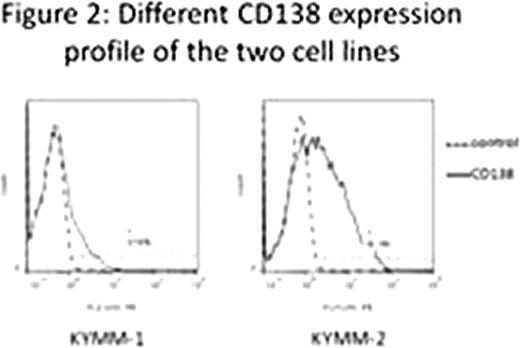
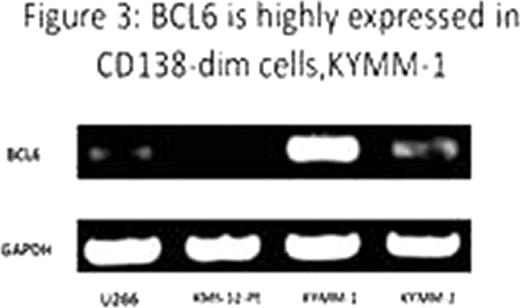
Flowcytometry analysis of primary MM cells revealed a significant decrease of CD138 expression in those with relapse or progressive diseases compared to untreated MM patients (p<0.05, Fisher's exact test). Mean survival duration of CD138-dim cases (1,039 days) was significantly shorter than that of untreated patients with high CD138 expression (2,321 days) (Figure 1, p<0.05, Log-rank test). Percent CD138 surface expression in KYMM-1 was 8.56, while that in KYMM-2 was 41.3, indicating expression of CD138 was differentially regulated in these two cell lines despite these came from the same patient (Figure 2). CD138 mRNA expression was significantly less in KYMM-1 compared to U266 and KYMM-2, suggesting that CD138 was down-regulated at the transcriptional level. The expression of Blimp1 and IRF4, genes associated with plasma cell differentiation and highly expressed in plasma cells and MM cells, was low in KYMM-1 than in KYMM-2, suggesting an aberrant differentiation state in a CD138-dim MM cell line. No aberrant methylation was observed in the promoter lesion of CD138, IRF4 and Blimp-1. High expression of BCL6 in KYMM-1, which is a repressor of IRF4 and Blimp-1, was found (Figure 3).
Our observations suggest that low CD138 expression in MM cells could serve as an indicator for poor prognosis. Since reduced CD138 expression is preferentially seen in previously treated cases, the regulation of CD138 gene could be associated with disease progression or drug resistance. Previous reports have showed the significance of BCL6 in MM (Hideshima et al. Blood.2010; 115 (18): 3772–3775. Fuhler GM et al. Exp Cell Res. 2010; 316(11):1816–28). High expression of BCL6 in KYMM-1 suggests immature phenotype resulted from down-regulation of IRF4 and Blimp-1 by BCL6. The observed distinctly different expression profile of transcription factors regulating the differentiation of plasma cells in the two cell lines suggest that a transition of differentiation state may have occurred during disease progression.
No relevant conflicts of interest to declare.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal