Abstract
Abstract 3877
B-cell chronic lymphocytic leukemia (B-CLL) is characterized by variable clinical presentation with different involvement of various lymphoid compartments i.e. peripheral blood, bone marrow and lymphoid organs such as lymph nodes and spleen. Also there is a well documented intraclonal and interclonal variability of B-CLL cells in different microenvironments regarding a number of surface and intracellular molecules (for example CD38 and ZAP-70). This variable distribution of tumor mass has strong association with prognosis and a well documented influence on response to antibodies like rituximab and alemtuzumab. There is a well documented efficacy of rituximab in cases with with 11q deletion (associated with significant lymphadenopathy), and known resistance to alemtuzumab in pateints with bulky lymphadenopaty (>5cm).
Aim of this study was to evaluate level of expression of CD20 and CD52 along with key chemokine receptor CXCR-4 and proliferation marker Ki-67 on B-CLL lymphocytes and intra and interclonal differences dependent on different microenvironment, ie peripheral blood (PB), bone marrow (BM) and lymph nodes (LN).
PB, BM and LN samples were taken by conventional techniques (venepuncture and fine needle aspiration) on the same day. The expression levels of CD20, CD52, CXCR-4 and Ki-67 molecules on CD19+CD5+ B-CLL cells were analyzed by flow cytometry. Results were expressed as mean fluorescence intensity (MFI) and analyzed by paired tests.
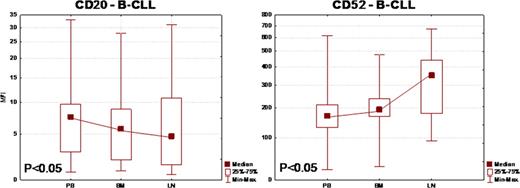
We have analyzed samples taken from 25 typical B-CLL patients with median age of 72 years. There were 14 males and 11 females. Mean beta-2 microglobulin was 4.3mg/l, mean TTM size was 9.8 and mean TD was 0.74. There were 13, 5 and 7 patients in Binet stage A, B and C, respectively. There were 4 previously treated patients (patients were not treated 3 months before sampling) of whom one patient was previously treated with both rituximab and alemtuzumab. Among included patients there were patients with 11q deletion and with 17p deletion. Median expression level (MFI) of CD52 on B-CLL cells was 171, 193, and 352 for PB, BM and LN respectively (p<0.05) and on T cells was 224, 171 and 137 (p<0.05). Median expression level (MFI) of CD20 on B-CLL cells was 7.5, 5.7 and 4.7 for PB, BM and LN respectively (p<0.05). These results were very consistent in this clinically and cytogenetically heterogenous group of B-CLL patients showing the same pattern in almost all patients. Median expression level (MFI) of CXCR-4 was 9.6, 5.9 and 2.6 (p<0.05) and Ki-67 was 1.08, 1.27 and 1.64 (p<0.05) for PB, BM and LN respectively. There was no correlation of CXCR-4 with CD20 and C52 expression in any compartment and there is positive correlation of Ki-67 with both CD20 and CD52 in PB but not in other compartments.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal