Abstract
Abstract 3841
The efficacy of azacitidine in the treatment of high risk myelodysplastic syndromes (MDS), chronic myelomonocytic leukaemia (CMML) and acute myeloid leukaemia (AML) (20–30% blasts) has been demonstrated. To investigate the efficacy of azacitidine in daily clinical practice and to identify predictors for response, we analyzed a cohort of 90 MDS, CMML and AML patients who have been treated in a Dutch compassionate patient named program.
Patients received azacitidine for a median of 5 cycles (range 1–19). The overall response rate (CR/PR/HI) was 57% in low risk MDS, 53% in high risk MDS, 50% in CMML, and 39% in AML patients. Median overall survival (OS) was 13.0 (9.8–16.2) months. In multivariate analysis we confirmed that circulating blasts (HR 0.48, 95% CI 0.24–0.99; p=.05) and poor risk cytogenetics (HR 0.45, 95% CI 0.22–0.91; p=.03) are independent predictors for OS. Interestingly, in this analysis we also identified platelet doubling after the first cycle of azacitidine as a simple and independent positive predictor for OS (HR 5.4, 95% CI 0.73–39.9; p=.10).
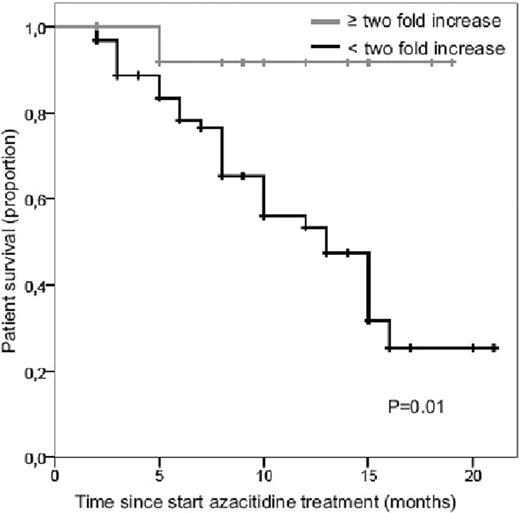
Of the 90 treated patients, 14 (16%) had an at least two-fold increase in platelet count after the first cycle of azacitidine, which was associated with significant better OS (p=.01, according to logrank test) (figure). Of these 14 patients 13 could be classified according the azacitidine prognostic scoring system for OS as recently proposed by Itzykson et al. (Blood:2011;117:403); 6 patients belonged to the low risk and 7 to the intermediate risk group. Median baseline platelet count of these patients was 35 x109/L (range 2–290 x109/L). Characteristics of this subgroup of patients were not significantly different from the patients without platelet doubling. Interestingly, platelet doubling was observed in all cytogenetic risk groups, in patients with and without circulating blasts, and in patients who are transfusion dependent and independent.
Wijermans:Centocor Ortho Biotech Research & Development: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal