Abstract
Abstract 3772
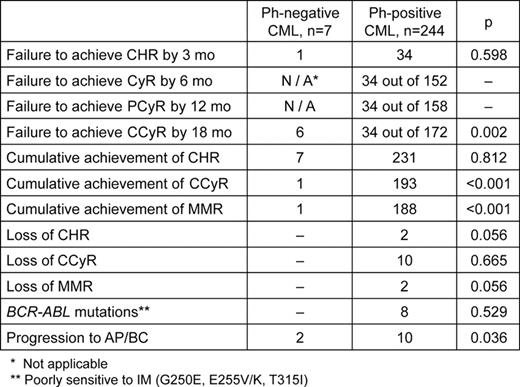
Around 1–2% of the CML patients do not have t(9;22)(q34;q11) detectable by conventional cytogenetics, but carry BCR-ABL fusion revealed by fluorescence in situ hybridization (FISH) and/or reverse-transcriptase PCR (RT-PCR). Outcome of Imatinib (IM) treatment in this group of patients remains unclear. Aim. To evaluate IM treatment efficacy in Ph-negative BCR-ABL- positive CML patients in comparison with Ph-positive ones. Methods. Initial diagnostics including chromosome banding analysis (CBA) and RT-PCR was performed in 251 CML patients who received 400 or 600 mg IM once a day. This group consisted of 181 patients in early chronic phase (CP), 65 patients in late CP and 4 patients in accelerated phase (AP). CBA was done after 24 hours culture. G-banding was performed by a trypsin-Giemsa method. Karyotypes were described according to ISCN (2005). At least 20 metaphases for each sample were analyzed. FISH assay using Dual-Colour Dual-Fusion BCR-ABL Translocation Probe (Abbott, USA) was applied on at least 200 interphase nuclei (I-FISH) and on all available metaphases. CBA and FISH were performed at the time of diagnostics and every 6 months of IM treatment. Cytogenetic response (CyR), partial cytogenetic response (PCyR) and complete cytogenetic response (CCyR) in Ph-positive CML patients were evaluated by CBA in contrast to Ph-negative patients those were assessed by I-FISH only. In Ph-negative CML patients CCyR were assumed as less than 1% of BCR-ABL -positive nuclei (N. Testoni et al, Blood, 2009). Quantitative measurement of BCR-ABL/ABL transcripts ratio by real-time quantitative PCR (RQ-PCR) was done every 3–6 months. Detection of point mutations in the BCR-ABL tyrosine kinase domain (TKD) was performed by direct sequencing of RT-PCR products. According to European LeukemiaNet recommendations (M. Baccarani et al, JCO, 2009), failures were defined as no complete hematological response (CHR) at 3 months, no CyR at 6 months, less that PCyR at 12 mo, less than CCyR at 18 months, loss of CHR, CCyR or major molecular response (MMR) at any time during treatment, newly acquired BCR-ABL mutation poorly sensitive to IM (G250E, E255V/K, T315I), or progression to the AP and/or blast crisis (BC). In Ph-negative patients 6 months and 12 months time-points, when CyR and PCyR have to be estimated, were excluded from the analysis due to lack of direct correlation between percentage of BCR-ABL -positive nuclei and CBA data (N. Testoni et al, Blood, 2009). Failure-free survival (FFS) and overall survival (OS) were calculated separately for Ph-positive and Ph-negative patients' groups. Results. In respect to t(9;22)(q34;q11) detection by CBA at the time of initial diagnosis all CML patients were divided into 2 groups: Ph-positive (n=244) and Ph-negative (n=7). In spite of presence of normal karyotype, all patients in the second group harboured BCR-ABL fusion gene revealed by FISH and RT-PCR. Groups were not significantly different in age at diagnosis, sex, Sokal risk group distribution, stage of disease at the time of IM treatment beginning, types of BCR-ABL fusion transcripts. Median time of follow-up was 43 months (range 26–66) in Ph-negative group and 36 months (range 18–105) in Ph-positive. Treatment results are shown in table. Although 6 of 7 Ph-negative CML patients achieved CHR by 3 months, only 1 of them was in CCyR by 18 mo, that was significantly lower than in Ph-positive group (p<0.001). Moreover, Ph-negative patients had high percentage of BCR-ABL -positive nuclei in BM both at 12 months (median 35% (range 1–60%)) and at 18 months of IM therapy (median 49% (range 0–92%)). Two Ph-negative CML patients progressed to AP and died subsequently. None of Ph-negative patients had BCR-ABL mutations, duplication or amplification. FFS in Ph-negative CML patients treated by IM was significantly lower than in 244 Ph-positive ones 0.14±0.13 vs 0.62±0.03 (p=0.007), while OS was comparable: 0.70±0.15 vs 0.85±0.02, (p=0.47), respectively. Conclusions. In our series treatment outcomes in Ph-negative CML patients who received IM at a dose of 400 or 600 mg once daily were significantly worse in comparison with Ph-positive ones. However, dose escalation or switching to second-generation TKI prevented further disease progression or CML-related deaths. Resistance in the observed group seems to have BCR-ABL-independent mechanisms.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal