Abstract
Abstract 3759
Imatinib treatment dramatically improves survival in chronic myeloid leukemia (CML) patients, but whether its effects of imatinib can be considered a cure remains controversial. This is because primitive, quiescent, Philadelphia-positive stem cells from patients with CML are insensitive to imatinib in vitro. Nonetheless, it was recently recognized that some patients with a complete molecular response (CMR) could sustain that response after discontinuation of imatinib. In a non-randomized prospective study, Mahon et al. reported that among patients with CMR lasting at least 2 years, CMR was sustained in 41% after discontinuation of imatinib.
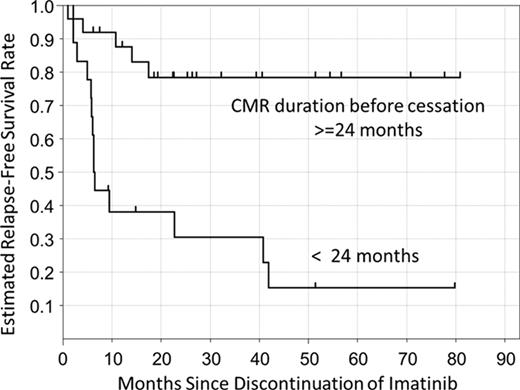
To characterize the clinical outcomes and profiles of chronic phase CML patients who discontinued imatinib, we conducted a nationwide survey in Japan. Among 3,242 imatinib-treated CML patients, 50 (1.5%) were identified who discontinued imatinib therapy for at least 6 months; of those, 43 were analyzed further. Molecular recurrence was detected in 19 patients, and the complete molecular response (CMR) rate was estimated to be 47% following imatinib discontinuation. Notably, the durations of imatinib therapy and CMR before cessation of therapy were significantly longer, and imatinib dose intensity and the frequency of prior IFN-a administration were significantly higher, in patients sustaining CMR for 12 months after cessation than in those with molecular recurrence. No significant correlations were detected between molecular recurrence and age, sex, Sokal risk, imatinib daily dose, combination with IFN-a, or time to achieve CMR. Moreover, we found a significant difference in estimated CMR rate following discontinuation between patients who had sustained CMR for greater than 24 months prior to imatinib discontinuation and those with less than 24 months (78% vs. 15%, p =0.0002 by Log-rank test, Figure). Based on multivariate regression analysis, only imatinib dose intensity and prior IFN-a administration were independently predictive of molecular recurrence within 12 months (p =0.0035, p =0.0060). The identified prediction formula was: Y= −0.0061 x dose intensity of imatinib(g)-3.17171 x prior IFN-a(Yes=1/No=0) +4.0124. If 1/(1+exp(-1 × Y)) > 0.5, molecular recurrence was predicted; the total accuracy rate of the formula was 82.5%.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal