Abstract
Abstract 3720
A Phase I study revealed that vaccination of cancer patients with irradiated autologous tumor cells and GM.CD40L bystander cells (engineered to secrete GM-CSF and express CD40L) is safe, recruits/activates dendritic cells, and elicits tumor-specific T cell responses. We report the long term followup using this vaccine strategy in patients with MCL, an aggressive and incurable B-cell malignancy.
After lymph node resection for autologous tumor harvest, 4–6 cycles of chemotherapy, and restaging (CT, endoscopy, bone marrow biopsy), patients with usable vaccine who achieved a PR or CR lasting 1 month were vaccinated x4 at 28-day intervals with IL-2 (0.5 × 106 U SC BID × 14 d/cycle). Patients were monitored for toxicity, tumor response, tumor-specific immune responses, and EFS/OS.
43 were enrolled, including 21 with relapsed MCL, 20 with int/high MIPI, and 6 with blastoid MCL. Twenty never received vaccine: 2 withdrew consent, 1 progressed rapidly prior to lymph node harvest, 7 had insufficient or contaminated specimens, and 10 progressed/died during chemotherapy. The unvaccinated were older (68.4y vs 62.8y; p=.026) but otherwise did not differ significantly by stage, LDH, MIPI, de novo status, or number of prior treatments.
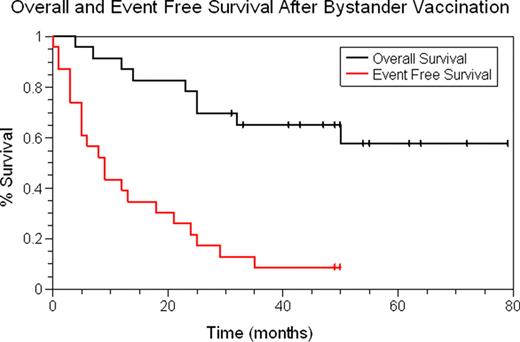
Among 23 treated, 10 had relapsed disease, 10 had an int/high MIPI, and 2 had blastoid MCL. Pre-vaccination response following chemotherapy included 7 CR, 15 PR, and one SD. At 6 months after vaccination, 2 pts in PR had resolution of MRD within the bone marrow, 10 progressed (including 3 who progressed after only 1–2 vaccines), and 11 had no change. Two are still in CR at 49 & 50 months. Median EFS and OS are calculated from receipt of first vaccine. Median EFS is 9 mo, however, this high risk cohort continues to show a prolonged median OS, which has not yet been reached (median follow-up 43 mo, range 4–79 mo). An increase in interferon gamma secretion as measured by ELISPOT assays was observed in 15 of the 23 patients (65%). Additionally, this was accompanied by an increase in EFS among those with a positive response.
These phase 2 data are analogous to what we have seen among 4 patients with relapsed MCL treated on our phase 1 trial (EFS 6.5 mo, OS 82 mo), and support the need for further studies using GM.CD40L bystander vaccination. Similar discrepancies in EFS and OS have also been observed with other approved immunotherapeutics, ipilumumab & sipleucel-T (Hodi et al. 2010, Kantoff et al. 2010).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal