Abstract
Obinutuzumab (GA101) is a type II glycoengineered, humanized anti-CD20 monoclonal antibody that has increased antibody-dependent cellular cytotoxicity and direct cell death activity but lower complement-dependent cytotoxicity compared with type I anti-CD20 antibodies such as rituximab and ofatumumab. GA101 is in clinical development for the treatment of lymphoma and chronic lymphocytic leukemia. The Phase I/II study BO20999 has evaluated the efficacy and safety of GA101 monotherapy in patients with relapsed/refractory aggressive non-Hodgkin's lymphoma (aNHL). Here, we report updated Phase II results including progression-free survival (PFS) and best overall response (BOR).
Patients (n = 40) were randomized to receive GA101 (D1, D8 and D22, then 3-weekly for total of 9 infusions) at either a high dose (1,600 mg on D1 and D8, then 800 mg thereafter; 1,600/800 mg cohort; n = 19), or a flat low dose of 400 mg (400/400 mg cohort; n = 21).
Baseline patient characteristics
| Characteristic . | 400/400 mg (n = 21) . | 1,600/800 mg (n = 19) . | All (n = 40) . |
|---|---|---|---|
| Median age, years (range) | 70 (43–80) | 72 (22–85) | 71 (22–85) |
| Histology, n | |||
| DLBCL | 10 | 15 | 25 |
| MCL | 11 | 4 | 15 |
| Median number of prior treatments, n (range) | 4 (1–17) | 3 (1–6) | 3 (1–17) |
| Previous rituximab, n | 21 | 19 | 40 |
| Rituximab refractory*, n | 13 | 12 | 25 |
| Prior stem cell transplant, n | 2 | 6 | 8 |
| Characteristic . | 400/400 mg (n = 21) . | 1,600/800 mg (n = 19) . | All (n = 40) . |
|---|---|---|---|
| Median age, years (range) | 70 (43–80) | 72 (22–85) | 71 (22–85) |
| Histology, n | |||
| DLBCL | 10 | 15 | 25 |
| MCL | 11 | 4 | 15 |
| Median number of prior treatments, n (range) | 4 (1–17) | 3 (1–6) | 3 (1–17) |
| Previous rituximab, n | 21 | 19 | 40 |
| Rituximab refractory*, n | 13 | 12 | 25 |
| Prior stem cell transplant, n | 2 | 6 | 8 |
DLBCL, diffuse large B-cell lymphoma; MCL, mantle cell lymphoma.
Rituximab refractory defined as patients who had a response of < 6 months or who failed to respond to a rituximab-containing regimen (rituximab monotherapy or in combination with chemotherapy) at any point during their treatment history.
Best overall response according to diagnosis and cohort
| . | DLBCL . | MCL . | ||
|---|---|---|---|---|
| Response, n (%) . | 400/400 mg (n = 10) . | 1,600/800 mg (n = 15) . | 400/400 mg (n = 11) . | 1,600/800 mg (n = 4) . |
| Complete response (CR) | 0 (0.0) | 3 (20.0) | 2 (18.2) | 0 (0.0) |
| CR unconfirmed | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Partial response | 2 (20.0) | 2 (13.3) | 0 (0.0) | 2 (50.0) |
| Stable disease | 1 (10.0) | 1 (6.7) | 3 (27.3) | 0 (0.0) |
| Progressive disease | 5 (50.0) | 9 (60.0) | 6 (54.5) | 2 (50.0) |
| No response assessment | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| . | DLBCL . | MCL . | ||
|---|---|---|---|---|
| Response, n (%) . | 400/400 mg (n = 10) . | 1,600/800 mg (n = 15) . | 400/400 mg (n = 11) . | 1,600/800 mg (n = 4) . |
| Complete response (CR) | 0 (0.0) | 3 (20.0) | 2 (18.2) | 0 (0.0) |
| CR unconfirmed | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Partial response | 2 (20.0) | 2 (13.3) | 0 (0.0) | 2 (50.0) |
| Stable disease | 1 (10.0) | 1 (6.7) | 3 (27.3) | 0 (0.0) |
| Progressive disease | 5 (50.0) | 9 (60.0) | 6 (54.5) | 2 (50.0) |
| No response assessment | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
DLBCL, diffuse large B-cell lymphoma; MCL, mantle cell lymphoma.
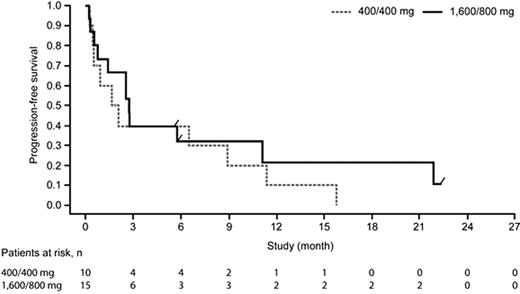
Progression-free survival for patients with diffuse large B-cell lymphoma
In conclusion, GA101 shows encouraging single-agent efficacy in these heavily pretreated patients with relapsed/refractory aNHL (DLBCL or MCL). A Phase III trial of rituximab plus CHOP vs GA101 plus CHOP in first-line DLBCL has recently started.
Morschhauser:Roche: Honoraria; Celgene: Consultancy, Honoraria. Cartron:Roche: Consultancy, Honoraria; GSK: Honoraria; LFB: Honoraria. Milpied:Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees. Wenger:Roche: Employment. Wassner-Fritsch:Roche: Employment. Asikanius:Roche: Employment. Salles:Roche: Consultancy, Honoraria.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal