Abstract
Abstract 3631
Despite improvements in clinical outcome with intensification of induction and consolidation chemotherapy, the majority of patients with AML will ultimately relapse. At first relapse, the European Prognostic Index (EPI) stratifies outcome according to relapse-free interval, karyotype, age and prior allogeneic stem cell transplant (Breems et al, JCO 2005; 1969). Recently, FLT3-ITD has also emerged as a predictor of poor outcome in relapsed patients (Chevallier et al, Leukemia 2011; 939). The purpose of this study was to define predictors of outcome in patients with AML treated at first relapse with FLAG-Amsacrine (Fludarabine 30mg/m2/day days 1–5; Cytarabine 2g/m2/day days 1–5; G-CSF 300mcg/day days 1–6; Amsacrine 100mg/m2/day days 1–3), particularly in the context of FLT3-ITD status and prior high-dose ara-C (HiDAC) exposure.
Patients treated at The Alfred, Box Hill, and Geelong hospitals with FLAG-Amsacrine between 2002 and 2011 were retrospectively identified. Statistical analysis of clinical outcomes related to toxicity, response and survival was performed with SPSS™ and GraphPad Prism™.
56 patients with AML in first relapse (28 male, 28 female), median age 50.5 years (range 18–70), received FLAG-Amsacrine as salvage therapy. The patient characteristics are summarised in Table 1. 48 patients had a history of prior HiDAC-exposure and 11 (19.7%) were known to be FLT3-ITD positive. The median time to neutrophil and platelet recovery in those attaining CR was 28.5 and 32 days respectively. 42-day treatment related mortality was 12.5%.
Patient Characteristics
| Prognostic Factor . | n (%) . |
|---|---|
| European Prognostic Index Category (age <60) | |
| Favourable | 2 (5.2%) |
| Intermediate | 18 (47.4%) |
| Poor | 18 (47.4%) |
| HiDAC exposure | |
| Previous HiDAC | 48 (85.7%) |
| No Previous HiDAC | 8 (14.3%) |
| FLT3-ITD status | |
| Positive | 11 (19.7%) |
| Negative | 25 (44.6%) |
| Unknown | 20 (35.7%) |
| Prognostic Factor . | n (%) . |
|---|---|
| European Prognostic Index Category (age <60) | |
| Favourable | 2 (5.2%) |
| Intermediate | 18 (47.4%) |
| Poor | 18 (47.4%) |
| HiDAC exposure | |
| Previous HiDAC | 48 (85.7%) |
| No Previous HiDAC | 8 (14.3%) |
| FLT3-ITD status | |
| Positive | 11 (19.7%) |
| Negative | 25 (44.6%) |
| Unknown | 20 (35.7%) |
The overall CR/CRi rate after FLAG-Amsacrine was 61% with 27% refractory to treatment. Median EFS (censored at the time of subsequent allograft) and OS from treatment commencement was 6.7 and 10.6 months respectively (median follow up 6.5 months; range 1–55 months).
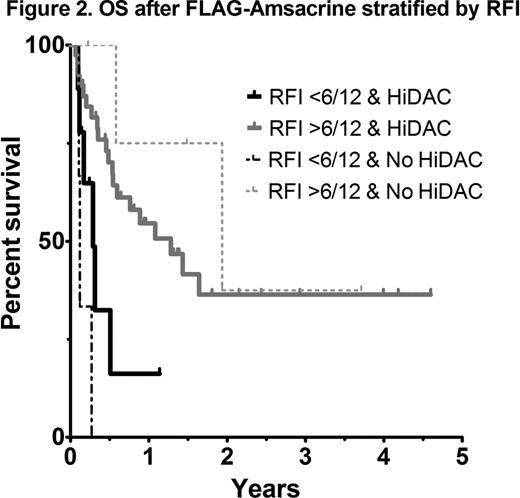
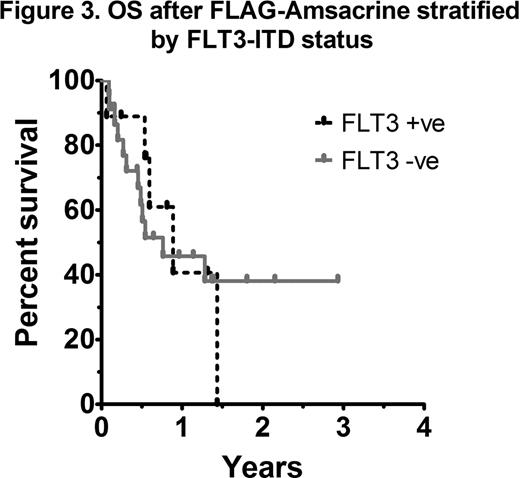
As most patients with AML receive HiDAC at some stage during induction or consolidation, analysis of outcomes according to the EPI score in those with prior HiDAC exposure was performed. A statistically significant difference in OS was not observed between intermediate and poor risk EPI groups (41 vs 42% 1yr OS respectively; p=0.74) (Figure 1). An analysis of each EPI risk determinant showed that a relapse-free interval (RFI) of less than 6 months was the most important factor influencing overall survival (4 vs 17 months, p=0.03). In patients with early relapse (RFI <6 months) treated with FLAG-Amsacrine, survival was dismal, even in those not previously exposed to HiDAC (Figure 2). Adverse risk karyotype (p=0.22), prior allograft (p=0.92) and age >60 (p=0.91) did not negatively impact on OS after FLAG-Amsacrine. There was no significant difference in the CR/CRi rate (60 vs 62.5%, p=1.0), EFS (p=0.33) or OS (p=0.81) of patients who had received FLAG-Amsacrine following prior HiDAC based therapy compared with those who had not received HiDAC. A significant difference in OS was not observed in relation to FLT3-ITD status (p=0.86; Figure 3).
22 patients went on to allogeneic stem cell transplantation with a median time to allograft of 83.5 days (range 29–340 days). Patients who received an allograft had longer median OS (not reached vs 4 months, p=0.01). 16 patients remain in continuous CR, with a median remission duration of 13 months (range 3–45 months).
FLAG-Amsacrine is a tolerable and effective salvage regimen for AML in first relapse and represents an effective bridge to transplantation. Alternative investigational approaches should be considered for patients with very short duration of first remission, where outcomes after FLAG-Amsacrine were poor, even in the absence of prior HiDAC-based therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal