Abstract
Abstract 3605
Although a majority of patients with AML achieve complete response (CR) following 1 or 2 cycles of induction chemotherapy, rates of relapse-free and overall survival remain poor. In the US, typically the decision to administer re-induction chemotherapy depends upon the degree of leukemic cell clearance from the bone marrow at 10–14 days after initial induction. In this single-institution study, we assessed patients who underwent double induction chemotherapy for AML in an attempt to delineate specific clinical variables that might influence outcomes and decisions to utilize re-induction chemotherapy.
Between 2004 and 2010, patients who received 2 courses of induction chemotherapy for AML at the H. Lee Moffitt Cancer Center were analyzed. Individual charts were reviewed. Chi square test was used to compare categorical variables in univariate analysis. Kaplan Meier estimates were used to calculate OS. Log rank test was used for comparison between the 2 groups and Cox regression analysis was used for multivariable analysis of survival. Binomial logistic regression was used for multivariate assessment of response rates. All analyses were conducted using SPSS version 19.0 software.
We identified 164 patients with previously untreated AML who underwent double-induction chemotherapy at our center, 127 of whom had residual blasts ≥ 10% following initial induction. Baseline characteristics (%): male:female (68%:32%), age less than:greater than 60 (57%:43%), adverse:non adverse karyotype (39%:58%), de-novo:secondary AML (66%:32%).
The majority (97%) initially received anthracycline + cytarabine (“7+3”) based induction chemotherapy. Second induction utilized a high-dose cytarabine based regimen in 65% of patients. Overall response rate (CR + CRi) was 62%. Median survival for the entire cohort was 13.3 months (95% CI 11.4–15.3). Univariate analysis of prognostic variables associated with response and survival are shown in Table 1.
:Univariate assessment of response and survival
| Variable . | N . | CR/CRi (%) . | p-value . | Median OS (mo) . | p-value . | |
|---|---|---|---|---|---|---|
| Age | <60 | 73 | 51.9% | 0.2 | 13.33 | 0.302 |
| ≥60 | 54 | 48.1% | 13.03 | |||
| AHD | No | 84 | 69.6% | 0.55 | 13.7 | 0.98 |
| Yes | 41 | 30.4% | 11.7 | |||
| Karoytype | non-adverse | 75 | 59.2% | 0.71 | 14.67 | 0.072 |
| adverse | 49 | 40.8% | 11.9 | |||
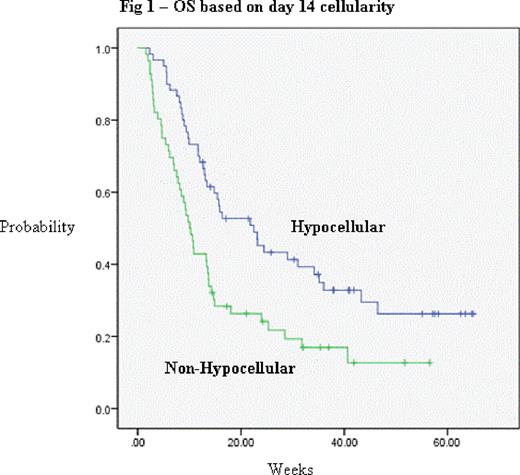
| D14 BM cell | Hypo | 60 | 78.3% | 0.002 | 22.5 | 0.002 |
| Non-hypo | 54 | 50% | 10.1 | |||
| Day 14 BM blast reduction | < 50% | 82 | 34.2% | 0.84 | 14.8 | 0.258 |
| ≥ 50% | 40 | 65.8% | 12.7 | |||
| Time of 2nd ind'n | ≤ 21d | 80 | 71.3% | 0.009 | 14.2 | 0.086 |
| ≥ 21d | 44 | 47.7% | 10.8 | |||
| 2nd induction | HDAC-based | 80 | 64.6% | 0.71 | 12.7 | 0.335 |
| Non-HDAC | 47 | 35.4% | 14.7 | |||
| Variable . | N . | CR/CRi (%) . | p-value . | Median OS (mo) . | p-value . | |
|---|---|---|---|---|---|---|
| Age | <60 | 73 | 51.9% | 0.2 | 13.33 | 0.302 |
| ≥60 | 54 | 48.1% | 13.03 | |||
| AHD | No | 84 | 69.6% | 0.55 | 13.7 | 0.98 |
| Yes | 41 | 30.4% | 11.7 | |||
| Karoytype | non-adverse | 75 | 59.2% | 0.71 | 14.67 | 0.072 |
| adverse | 49 | 40.8% | 11.9 | |||
| D14 BM cell | Hypo | 60 | 78.3% | 0.002 | 22.5 | 0.002 |
| Non-hypo | 54 | 50% | 10.1 | |||
| Day 14 BM blast reduction | < 50% | 82 | 34.2% | 0.84 | 14.8 | 0.258 |
| ≥ 50% | 40 | 65.8% | 12.7 | |||
| Time of 2nd ind'n | ≤ 21d | 80 | 71.3% | 0.009 | 14.2 | 0.086 |
| ≥ 21d | 44 | 47.7% | 10.8 | |||
| 2nd induction | HDAC-based | 80 | 64.6% | 0.71 | 12.7 | 0.335 |
| Non-HDAC | 47 | 35.4% | 14.7 | |||
On multivariate analysis, only adverse karyotype (p=0.02, HR 1.67) and non-hypocellular (≥ 20%) bone marrow at day 14 after 1st induction (p=0.002, HR 1.934) were statistically significant predictors of inferior survival (figure 1), but there was a trend toward inferior OS for re-induction beginning after day 21. The only statistically significant predictors for response (CR+CRi) in the logistic regression model were age < 60 (p=0.034, odds ratio 2.850) and hypocellular day 14 bone marrow after 1st induction (p <0.005, odds ratio 7.87).
In patients who received double induction therapy for AML, response was achieved in the majority, and the bone marrow cellularity at day 14 after induction cycle 1 was the strongest predictor of response and survival, strongly suggesting its consideration as a prognostic/stratification factor in future outcomes studies as well as studies testing new agents in refractory disease. Future analyses will include direct comparisons of outcomes and analysis of risk factors between patients receiving 1 versus 2 cycles of induction.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal