Abstract
Abstract 3542
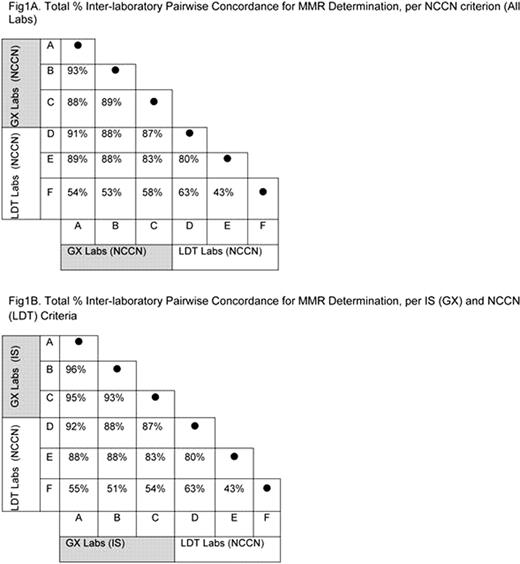
Achieving major molecular response (MMR) is an important milestone in chronic myeloid leukemia (CML) therapy. MMR has been defined as a 3-log reduction in BCR-ABL transcript levels from a standardized baseline (BL) established in the IRIS trial (Hughes TP, N Engl J Med. 2003). Standardization has been achieved through the development of an IS, which defines MMR as BCR-ABLIS = 0.1%. In contrast, the NCCN defines MMR as a 3-log reduction in BCR-ABL transcript levels but is indefinite on the definition of BL. Here, using reconstructed samples emulating CML patient BCR-ABL levels, the pairwise concordance of MMR determination was examined within and between 3 labs using the IS-standardized GeneXpert® (GX) system and 3 labs using laboratory-developed tests (LDTs). For comparative purposes, this analysis assumes BL is established at the time of diagnosis. Methods: 100 virtual patients (VPs) were emulated based on data from the REVEAL BCR-ABL Methods Comparison Study, in which 8 discrete levels of blinded K562 cell–spiked blood corresponding to BCR-ABLIS ratios ranging from ∼10% to ∼0.01% were analyzed by 3 labs using the IS-standardized GX system and 3 labs using non-IS LDTs. VP emulations were guided by actual patient outcomes in landmark analyses of 7- treatment response (Hughes TP, Blood. 2010). Treatment response profiles over an 18-month time horizon were modeled by assigning one of the 8 BCR-ABL levels ranging from approximately 10%-0.01% IS sampled in the REVEAL study to each of 4 virtual time points (eg, 3, 6, 12, and 18 months). BL levels were selected from quartiles representing pretreatment BCR- ABL ratios between 50–150%; results based on BL levels observed in the IRIS clinical trial will also be presented. 600 VP transcript profiles (VTPs) were then reconstructed using data from each of the 6 laboratories for all 100 VPs. The final 18-month time point in each VTP provided the BCR-ABL level against which the IS or NCCN objective criterion was applied to make MMR determinations. MMR concordance was evaluated by inspecting all possible inter-lab pairwise comparisons among the 100 VPs. Results: Pairwise concordance in MMR as determined by NCCN criterion among all 6 labs is shown in Fig 1A. MMR determinations among the 3 GX labs were concordant in 88% to 93% of VPs. In contrast, MMR determinations among the LDTs were concordant in 43% to 80% of VPs, and MMR determinations were concordant in 53% to 91% of VPs when compared between GX labs and LDTs. When MMR determination based on IS criterion for GX was considered, MMR concordance improved to 93% to 96% among the GX labs in contrast to 51% to 92% concordance observed between the GX and LDT sites (Fig 1B). It is noteworthy that Lab D results more closely approximated the IS than results from the other LDTs examined in the REVEAL study (data not shown). Although Lab D does not report results per the IS, it does report results relative to a median diagnostic BL, similar to the approach used in the IRIS trial. A healthcare system based on LDTs without any attempted IS standardization resulted in MMR concordance of only 43%. Potential sources of discordance among tests will be discussed in detail. Conclusions: These results illustrate that the NCCN criterion for MMR determination is not adequate for inter-lab comparisons of BCR-ABL transcript levels near the clinically important level of MMR. In contrast, standardization to the IS improves inter-lab concordance in MMR determination. Taken together, these results highlight the discrepancies that may result when comparing molecular responses between labs not standardized to the IS. As attainment of MMR is a critical milestone of CML therapy, errors in MMR determination may have an adverse impact on CML disease management.
Reddy:Novartis: Research Funding, as Presenting Author, sponsorship to attend ASH. Höfling:Novartis: Employment. Manning:Novartis: Employment. Mignault:Novartis: Employment. Mullaney:Novartis: Employment. Ossa:Novartis: Employment. Stein:Novartis: Employment. Wang:Novartis: Employment. Yang:Novartis: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal