Abstract
Abstract 3532
The study is to analyze the prognostic impact of post-induction-BM status combining minimal residual disease (MRD), neutrophil/monocyte maturation return to MDS and quantitation of hematogones in adult AML achieving first morphologic complete remission (mCR). The hypothesis is that the detection of aberrant myeloid progenitor cells, aberrant neutrophil/monocyte maturation and hematogones in post-induction BM by flow cytometry may predict the outcome of AML in mCR even without diagnostic specimen. The positive MRD and the aberrant neutrophil/monocyte maturation will predict worse prognosis. In contrast, positive hematogones will predict better prognosis.
Multidimensional flow cytometry was performed on bone marrow specimens from 47 consecutive non-M3 AML patients who had achieved mCR after standard 3+7 induction treatment. The in-remission immunophenotypic evaluation of BM was done before first consolidation treatment. The aberrant myeloid progenitor cells and aberrant neutrophil/monocyte maturation were defined by the flow cytometric scoring system (FCSS)1. Two investigators were blinded to corresponding pathologic, clinical or diagnostic flow cytometric data during initial analysis phase. After reaching agreement of the three parameters: aberrant myeloid progenitor cells (MRD ≥ 0.2% or not), aberrant neutrophil/monocyte maturation (FCSS ≥ 2 points or not) and hematogones (stages I / II B lymphoid progenitor cells ≥ 0.02% or not), those prognostic impacts on leukemia free survival (LFS) and overall survival (OS) were analyzed retrospectively.
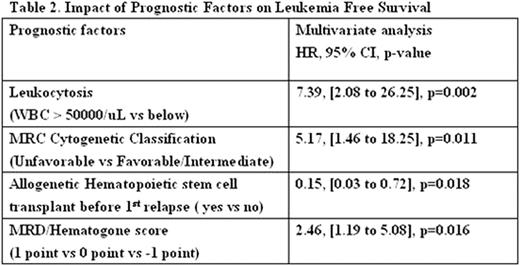
Table 1 summarizes the clinical characteristics of patients according to the status of MRD and hematogones. Nine (19%) patients who had MRD ≥0.2% had a significantly worse median LFS (9.0 months vs not reached; P =.006) and worse OS (24.0 months vs not reached; P =.059). Fourteen (30%) patients who had hematogone levels ≥ 0.02% had a significantly better median LFS (not reached vs 13.5 months; P =.045) and a trend of better OS (not reached vs 24.0 months; P =.070). Six (13%) patients who had FCSS ≥ 2 points had a worse median LFS (8.0 vs 48.0 months; P =.052) but not significantly worse OS (17.0 vs 28.0 months; P =.804). A Spearman coefficient for MRD ≥0.2% and hematogone levels ≥ 0.02% was -0.317 (P <.029), indicating a mildly negative correlation. However, MRD ≥0.2% and hematogones ≥ 0.02% were almost mutually exclusive. For clinical convenience, a MRD/Hematogone score was proposed that patient with MRD ≥0.2% was defined as 1 point and hematogone ≥ 0.02% was defined as -1 point. All patients could be categorized into three subgroups: 14(30%) patients with -1 point, 24(51%) patients with 0 point and 9(19%) with 1 point. Patients were stratified based on MRD/Hematogone score to assess survival using the Kaplan-Meier method and the Log-rank test. The median LFS were not reached, 48 months and 9 months for patients with -1 point, 0 point and 1 point, respectively (P =.015; Fig. 1 ). The median OS were not reached (-1 point), not reached (0 point) and 24 months (1 point) (P =.088). Multivariate analyses using Cox proportional hazard model demonstrated MRD/Hematogone score was an independent predictor of LFS (HR = 2.5, 95% CI, 1.2–5.1, P =.016; Table 2 ) after adjusting for MRC cytogenetic classification, WBC count (above or below 50000/uL) and whether undergoing allogenetic hematopoietic stem cell transplant before 1st relapse or not.
Even without a diagnostic specimen, flow cytometry can assess and quantify aberrant myeloid progenitor cells, aberrant neutrophil/monocyte maturation and hematogones in post-induction marrow. MRD ≥0.2% or neutrophil/monocyte abnormalities (FCSS ≥ 2) predicts shorter LFS. Hematogones ≥ 0.02% predicts longer LFS and correlates negatively with MRD ≥0.2%. Proposed MRD/Hematogone score for post-induction adult AML in mCR maybe offer a simple and practical risk assessment. And the score is worthy to be validated by prospective and larger scale study.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal