Abstract
Abstract 3489
Acute myeloid leukemia (AML) remains a difficult-to-treat disease, and outcome with standard conventional chemotherapeutics is often poor. An emerging approach uses monoclonal antibodies as a means to deliver targeted therapy. The antigen currently most exploited is CD33, in particular with gemtuzumab ozogamicin (GO), an immunoconjugate with a toxic calicheamicin derivative that causes DNA strand breaks, apoptosis, and cell death. However, while efficacious in some patients with untreated or relapsed/refractory disease, clinical response to GO is heterogeneous, emphasizing the need for studies to determine the subsets of patients that will benefit from GO. Although response to therapy is generally multi-factorial in origin, there is often a significant contribution of host genetic factors. Since GO targets CD33, we sought to determine the clinical impact of CD33 single nucleotide polymorphisms (SNPs) in a larger cohort of pediatric AML patients enrolled on Children's Oncology Group (COG) AAML03P1 pilot Phase III study, which explored the safety of GO addition to standard induction chemotherapy for newly-diagnosed AML.
We genotyped four CD33 SNPs [rs35112940 (G>A; Arg304Gly), rs12459419 (C>T; Ala14Val), rs2455069 (A>G; Arg69Gly), and rs1803254 (G>C; 3'UTR)] occurring with an allelic frequency greater than 10% in genomic DNA samples in 242 pediatric AML patients. These were treated on trial COG-AAML03P1, and determined the associations between CD33 SNPs with CD33 protein expression levels in diagnostic leukemic cells and clinical outcome.
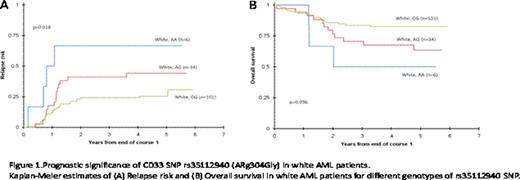
CD33 coding SNPs, rs35112940 (G>A; Arg304Gly) and rs12459419 (C>T; Ala14Val), and the 3'UTR SNP rs1803254 demonstrated significant associations with outcome or CD33 expression levels. Specifically, among white patients (which consisted of the major group 166/242=68.6%) homozygous for wild-type allele (GG) for the SNP rs35112940, a lower 3-year relapse risk from the end of course 1 was observed as compared to other genotypes (GG vs. AG vs. AA; 24±9% vs. 41±17% vs. 67±38%; p=0.018 Figure. 1A). Similarly, the 3-year overall survival from end of course 1 for among white patients homozygous for G allele in complete remission was 84±8% versus 68±15% for other genotypes (p=0.036; Figure. 1B). Patients homozygous for variant allele (TT) for SNP rs12459419 had a 3-year overall survival of 88±13% compared to 69±6% for other genotypes (p=0.056). Among white patients, the homozygous TT genotype also had a lower mean CD33 expression compared to other genotypes at this SNP (mean fluorescence intensity, 42 vs. 145, p=<0.001). Additionally, we observed an increased proportion of patients homozygous for variant allele (TT) for rs12459419 in the favorable risk group compared to genotypes CC and CT (52% vs. 31 %, p=0.034).
In conclusion, our findings suggest that CD33 SNPs are associated with clinical disease features such as CD33 expression levels and risk group as well as outcome after GO-containing chemotherapy in patients with newly diagnosed AML. The recently completed COG-AAML0531 randomized Phase III trial will allow validation of our findings. If their usefulness as a prognostic marker for treatment outcome of GO-based therapy is confirmed in an independent cohort, CD33 SNPs may serve to optimize the clinical use of this immunoconjugate; by extrapolation, prognostic information provided by CD33 SNPs may also be applicable to novel, second-generation CD33-targeting immuno-conjugates that have entered preclinical and clinical testing.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal