Abstract
Abstract 3418
Mesenchymal stem cells (MSCs) have the unique ability of homing to tumor tissue. Most of prior studies used xenograft models to demonstrate hMSC homing to human cancer on the immunodeficient mice. This model is far away from clinically relevant condition. It was also unknown whether MSCs target specific or all the tumor types in immunocompotent host as well as the mechanism driving MSCs home to tumor site.
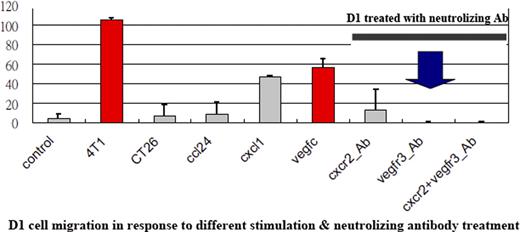
Migration assay was done to access the in vitro migration effect of D1 cells (BM-MSCs derived from balb/c mice) in response to 4T1, CT26, and Rag cells (breast cancer, colon cancer, and renal cancer cell line derived from balb/c mice). Firefly luciferase (Luc) stably expressed D1 (D1-Luc) was selected and maintained. 4T1 and CT26 cells were inoculated into 6 weeks balb/c mice. D1-Luc cells were then injected into normal or tumor-bearing balb/c through tail vein at different tumor stage (small tumors and big tumors). Besides, 4T1 and CT26 cells were inoculated into bilateral sides of balb/c mice. After tumor formation, D1-Luc cells were injected locally into one side to see whether the D1-Luc can migrate to the contralateral tumor or not. The in vivo tumor homing was accessed by IVIS (xenogen). Finally, 4T1 and CT26 tumors were excised from the mice to analyze gene expression profile (GEP). Chemokines/cytokines highly expressed on 4T1 but not CT26 were selected for blocking study in vitro and in vivo.
Both in vitro and in vivo studies showed D1 homing to 4T1 (breast) tumor only but not CT26 (colon) and Rag (renal) tumor. In case of 4T1, D1-Luc homed to all (100%) the tumor-bearing mice though tail vein injection. Besides, D1-Luc cells also home to both big and small 4T1 tumor simultaneously. When D1-Luc injected locally to one side of 4T1-bearing mice, the luciferase activity can be detected at the contralateral 4T1 tumor one hour after injection. In case of CT26, luciferase activity can not be detected on the contralateral CT26 tumor up to 7 days after injection. The GEP study showed VEGF-C, CCL24, CXCL 1/2/7 were highly expressed in 4T1 but not CT26. Blocking the VEGF-C receptor of D1 alone by using neutralizing antibody was sufficient to suppress D1 cells migration toward 4T1 tumor both in vitro and in vivo. The expression of VEGF-C is 100-fold higher in tumor tissue comparing to normal tissues (lung, liver, spleen, and kidney) by using RT-PCR.
MSCs home to specific tumor only and specific ligand-receptor relationship between MSCs and cancer cells determines the specificity. In this immunocompetent, syngeneic mice model, BM-MSCs homes to breast cancer but not normal tissue via VEGF-C and VEGF-C receptor axis. Our model also provides a good platform to the future development of MSC-based cell therapy. Before starting such a trial in human, it is essential to identify a specific ligand that is highly expressed on tumor (maybe very specific type of tumor) but not normal tissues and the MSCs should have corresponding receptor. This work was supported in part by the research grant from Taiwan National Science Council (NSC-96-3111-B-039-001), and Department of Health, China Medical University Hospital Cancer Research of Excellence (DOH-100-TD-C-111-005).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal