Abstract
Abstract 3359
Anticoagulation is one of the most widely used and essential clinical practices in modern medicine. Heparins are universally used for the prevention of blood coagulation in surgical procedures and for the treatment of diseases such as venous thromboembolism (VTE). Unfractionated heparin (UFH), low and ultra-low molecular weight heparins (LMWHs&ULMWHs) and synthetic pentasaccharides such as fondaparinux and idraparinux are the most commonly used clinical anticoagulants. Heparin is the second most widely used drug after insulin. However, it is associated with bleeding complications and heparin induced thrombocytopenia. Hence a careful monitoring and neutralization of heparins is essential. Protamine is the only clinically approved antidote to UFH, but it has several side effects and is not effective against LMWHs and synthetic pentasaccharides. Hence there is an unmet clinical need to develop safer and more efficient antidotes for all these anticoagulants. Here, we report a novel polymer based antidote, heparin binding synthetic polyvalent cationic macromolecule (HBSPCM), that completely neutralizes UFH and LMWHs in vitro and in vivo and is highly biocompatible and non-toxic in the required therapeutic dose range.
HBSPCMs were synthesized by the polymerization of glycidol and methoxy polyethylene glycol and functionalized with multifunctional tertiary amines as binding groups. Blood compatibility of HBSPCM was evaluated by activated partial thromboplastin time (APTT), prothrombin time (PT), thromboelastography (TEG), platelet and complement activation assays. Cell viability of HBSPCM was evaluated in human umbilical vein endothelial cells and fibroblast cells. Single dose tolerability in mice was studied by injecting escalating doses of HBSPCM and monitoring the body weights over a period of 29 days. HBSPCMs were tested for in vitro heparin neutralization by measuring the APTT in human plasma. An anti-fXa assay was used to study the in vivo neutralization of heparins by HBSPCM in a rat model. Pharmacokinetics and biodistribution of 3H-labeled HBSPCM was studied by bolus i.v. injection in female Balb/c mice and measuring the radioactivity in major organs at different time points.
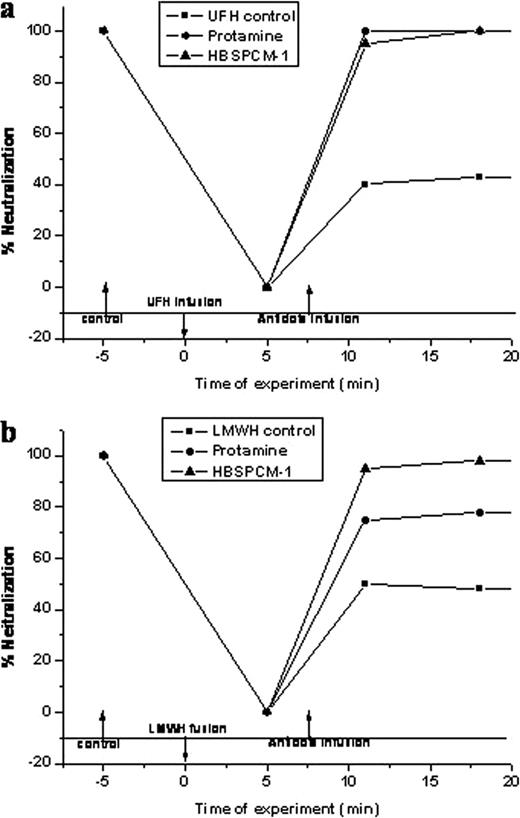
The newly designed antidotes, HBSPCMs, alone do not show any adverse effect on blood coagulation, platelet and complement activation and cytotoxicity that reveal their excellent blood and cell compatibilities. When injected in mice, HBSPCMs were well-tolerated up to the maximum injected dose of 200 mg/kg, which is ten-fold higher than the maximum tolerated dose of protamine (20 mg/kg) (Figure 1 ). HBSPCMs were 20-times more efficient than protamine for neutralizing heparins in vitro, and unlike protamine they do not show anticoagulant effect at higher concentrations. In vivo studies in rats revealed complete neutralization of both UFH and LMWHs by HBSPCMs, and the neutralization activities for LMWHs were significantly higher than that of protamine (Figure 2 ). HBSPCMs and their heparin complexes showed rapid clearance through urine, without significant accumulation in major organs.
Body weights of female Balb/c mice injected intravenously with HBSPCM (a) and protamine (b). No significant change in the body weights over 29 days post injection period was observed for HBSPCM. Acute toxicity was observed at 30 mg/Kg of protamine.
Body weights of female Balb/c mice injected intravenously with HBSPCM (a) and protamine (b). No significant change in the body weights over 29 days post injection period was observed for HBSPCM. Acute toxicity was observed at 30 mg/Kg of protamine.
Neutralization of UFH (a) and LMWH (b) in vivo (in rat model) by HBSPCM and protamine. Rats were injected with 25 U of UFH or LMWH and blood was collected after at 5 min post injection. HBSPCM (20 mg/Kg) or protamine (3 mg/Kg) was injected at 7 min, and blood was collected at different time points and analyzed for fXa levels.
Neutralization of UFH (a) and LMWH (b) in vivo (in rat model) by HBSPCM and protamine. Rats were injected with 25 U of UFH or LMWH and blood was collected after at 5 min post injection. HBSPCM (20 mg/Kg) or protamine (3 mg/Kg) was injected at 7 min, and blood was collected at different time points and analyzed for fXa levels.
In order to overcome the challenges associated with heparin based anticoagulation therapy, there is an increasing demand to develop safer, stable, effective, economical and universal antidotes which could neutralize all the available heparin anticoagulants. The developed polymer based antidote, HBSPCM, represents a major breakthrough towards this goal and could be a potential replacement for protamine. This polymer based therapeutic agent opens the scope for the development of non-toxic antidotes for all heparin based drugs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal