Abstract
Abstract 3344
Combined oral contraceptives (COC) containing estrogens and progestins are associated with a 2 to 4-fold increased risk of venous thromboembolic events. Progestin-only contraceptives are commonly prescribed to women with a higher risk for thrombosis, although a lower thrombotic risk than COC has not been clearly established. We performed a systematic review and meta-analysis of published studies to estimate the risk ratio of venous thromboembolism (VTE) in women taking progesterone for the purpose of contraception.
We performed a literature search of cohort, case-control and randomized studies including women treated with progestin-only contraception compared with a no-hormone control group. All administration routes (oral, injectable and intra-uterine) were considered. The primary endpoint was the corrected risk ratio of VTE analyzed using a random effects model.
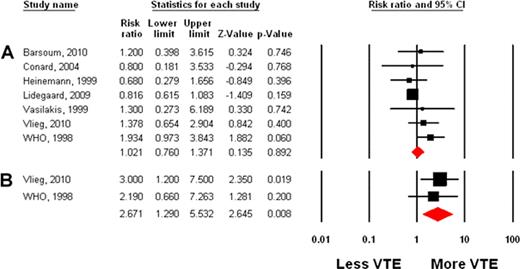
1150 references were retrieved; 7 studies were retained for analysis. The summary estimate of the adjusted risk ratio of VTE for women taking a progestin for contraception versus no hormone was 1.02 (95% CI=0.76–1.37, p=0.89). Heterogeneity between studies was minimal (Q-value=7.1, I2=15.49 and p=0.31). An analysis combining the unadjusted odds ratios of VTE gave a similar result (OR=1.24, 95% CI=0.93–1.66, p=0.14). Analysis by subgroup suggests a higher risk of thrombotic events for individuals using an injectable progestin, with an estimated risk ratio of VTE equal to 2.67 (95% CI=1.29–5.53, p=0.008), compared to 0.87 (95% CI=0.53–1.42, p=0.58) for oral administration.
Progestin-only oral formulations used for contraception do not appear to increase the risk of venous thromboembolic disease, although injectable administration was associated with an increased incidence of VTE relative to women not taking hormones that is comparable to the risk associated with COC.
| Study . | Format . | Drug Formulation(s) . | Number of Patients . | Adjusted Risk Ratio of VTE (95% CI)‡ . | |||
|---|---|---|---|---|---|---|---|
| Progestin . | No Hormone . | ||||||
| VTE . | No VTE . | VTE . | No VTE . | ||||
| 1. Barsoum et al, Thromb Res 2010 | case-control | O, I | 3 | 2 | 98 | 133 | 1.20 (0.40–3.62) |
| 2. Conard et al, Contraception 2004 | retrospective cohort | O | 3 | 99 | 6 | 96 | 0.80 (0.18–3.53) |
| 3. Heinemann et al, Eur J Contracept Reprod Health Care 1999 | case control | O | 7 | 54 | 174 | 1346 | 0.68 (0.28–1.66) |
| 4. Lidegaard et al, BMJ 2009 | retrospective cohort | O,U | 49 | N/A* | 2168 | N/A† | 0.82 (0.62–1.08) |
| 5. Vasilakis et al, Lancet 1999 | case-control | O, I | 2 | 26 | 13 | 161 | 1.30 (0.27–6.19) |
| 6. Vlieg et al, Arterioscler Thromb Vasc Bio 2010 | case-control | I, U | 23 | 41 | 421 | 1102 | 1.38 (0.65–2.90) |
| 7. WHO, Contraception 1998 | case-control | O, I | 32 | 98 | 635 | 2288 | 1.93 (0.97–3.84) |
| Study . | Format . | Drug Formulation(s) . | Number of Patients . | Adjusted Risk Ratio of VTE (95% CI)‡ . | |||
|---|---|---|---|---|---|---|---|
| Progestin . | No Hormone . | ||||||
| VTE . | No VTE . | VTE . | No VTE . | ||||
| 1. Barsoum et al, Thromb Res 2010 | case-control | O, I | 3 | 2 | 98 | 133 | 1.20 (0.40–3.62) |
| 2. Conard et al, Contraception 2004 | retrospective cohort | O | 3 | 99 | 6 | 96 | 0.80 (0.18–3.53) |
| 3. Heinemann et al, Eur J Contracept Reprod Health Care 1999 | case control | O | 7 | 54 | 174 | 1346 | 0.68 (0.28–1.66) |
| 4. Lidegaard et al, BMJ 2009 | retrospective cohort | O,U | 49 | N/A* | 2168 | N/A† | 0.82 (0.62–1.08) |
| 5. Vasilakis et al, Lancet 1999 | case-control | O, I | 2 | 26 | 13 | 161 | 1.30 (0.27–6.19) |
| 6. Vlieg et al, Arterioscler Thromb Vasc Bio 2010 | case-control | I, U | 23 | 41 | 421 | 1102 | 1.38 (0.65–2.90) |
| 7. WHO, Contraception 1998 | case-control | O, I | 32 | 98 | 635 | 2288 | 1.93 (0.97–3.84) |
Table Legend: O=oral, I=injectable, U=intra-uterine.
VTE incidence rate/10000 woman years =1.82 to 3.35, depending on the type of progestin.
VTE incidence rate/10000 woman years =3.01 for non-users.
Odds ratios and rate ratios were treated as risk ratios because of the low prevalence of disease.
Estimated risk ratio of VTE for progestin versus no hormone, using adjusted values; A, all routes of administration for all studies; B, injectable progestins only, for studies where those results were reported.
This publication was supported by Grant Number UL1 RR025752 from the National Center for Research Resources. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal