Abstract
Background/Methods: The relative safety and efficacy of romiplostim and rituximab in ITP have not been compared in clinical trials. In an open-label 52-week trial of SOC (N = 77) and romiplostim (N = 157) in ITP patients without prior splenectomy (Kuter et al, NEJM 2010), treatment failure was lower with romiplostim vs SOC (11% vs 30%, P<0.001). Within the SOC arm, 16 patients (21%) received rituximab; most (11/16) at QW × 4 (dose: 219–363 mg/m2), other rituximab dosing regimens varied considerably. We examine here outcomes of those 16 patients as compared with the romiplostim arm (in which 1 patient received rituximab) and the SOC arm as a whole.
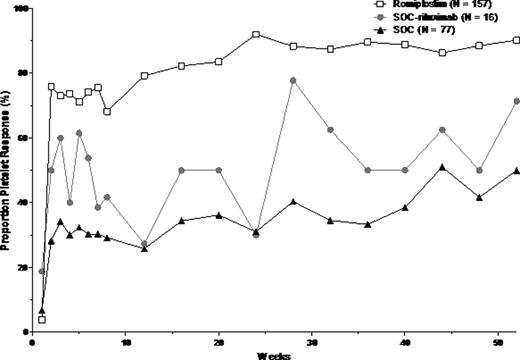
All 3 groups had comparable age, sex, and baseline platelet count (Table). Median ITP duration was somewhat shorter for romiplostim-treated patients and the SOC arm vs. SOC-rituximab patients (2.1, 2.3 vs 3.4 years). More romiplostim patients completed the 52-week treatment phase vs SOC-rituximab patients and the SOC arm (78% vs 44%, 56%). Fewer romiplostim patients vs. SOC-rituximab and SOC patients experienced treatment failure (4% vs 13%, 13%). Adverse events (AE), serious AE (SAE), treatment-related AE, and treatment-related SAE rates were similar for all 3 groups. Platelet response rates (platelet count >50×109/L, excluding 8 weeks after rescue medication) were higher with romiplostim than with SOC-rituximab or SOC as a whole (Graph).
When comparing concomitant ITP medications prior to rituximab and during or after rituximab treatment in the SOC-rituximab patients, there was no change in the proportion of patients receiving ITP medications (9/16 vs 10/16) or the number of medications per patient [median (range) of 1 (0, 3) vs 1 (0, 4)]. The most common prior to rituximab treatment were prednisone (7/16), IVIg (3/16) and anti-D (2/16); during or after rituximab treatment, the most common were prednisone (6/16), IVIg (4/16), anti-D (2/16), and methylprednisone (2/16).
This post hoc non-randomized comparison indicates that romiplostim may have greater effects on platelet responses than SOC-rituximab or SOC, with similar safety profiles. However, different treatment goals – ongoing use of romiplostim to maintain platelet response as opposed to one or more courses of rituximab which may result in a long-term response in the absence of other therapies (∼20% response rate at 5 years, Patel, ASH 2010) – makes efficacy comparisons problematic, particularly as the long-term response rate was not measured in this one-year study. Also not analyzed was how the use of concomitant ITP medications changed over the course of the study. As splenectomy and many immunosuppressive ITP medications are associated with significant morbidity and mortality over time, this is an important consideration. Most importantly, patients were selected for rituximab treatment by the investigators in a non-random way, as evidenced by the longer duration of ITP, making any generalizations difficult. Prospective controlled studies of romiplostim and rituximab in patients with ITP would be needed to provide further information.
| . | . | Romiplostim N = 157 . | SOC receiving rituximab N = 16 . | SOC N = 77 . |
|---|---|---|---|---|
| Baseline Characteristics | Age, median (range), years | 58 (18, 90) | 64.5 (29, 85) | 57 –86) |
| Female | 85 (54.1) | 10 (62.5) | 46 (59.7) | |
| ITP duration, median (range), years | 2.1 (0.02, 44.2) | 3.4 (0.01, 18.9) | 2.3 (0.01, 33.2) | |
| Platelet count (×109/L), mean (SD) | 30 (15) | 28 (20) | 25 (15) | |
| Disposition | Completed 52 weeks | 122 (78) | 7 (44) | 43 (56) |
| Discontinued due to: | 35 (22) | 9 (56) | 34 (44) | |
| –Didn't receive IP | 3 (2) | 0 | 2 (3) | |
| –Noncompliance | 1 (1) | 0 | 1 (1) | |
| –Adverse event | 7 (4) | 0 | 2 (3) | |
| –Withdrew consent | 4 (3) | 0 | 4 (5) | |
| –Subject request | 3 (2) | 0 | 0 | |
| –Alternative therapy | 6 (4) | 4 (25) | 14 (18) | |
| –Administrative decision | 1 (1) | 0 | 0 | |
| –Death | 1 (1) | 1 (6) | 1 (1) | |
| –Othera | 9 (6) | 4 (25) | 10 (13) | |
| Efficacy | Had splenectomy | 2 (1) | 5 (31) | 15 (20) |
| Had treatment failure, defined as ≥1 of the following | 6 (4) | 2 (13) | 10 (13) | |
| –Lack of efficacy | 2 (1) | 2 (13) | 4 (5) | |
| –Major bleeding | 3 (2) | 0 | 6 (8) | |
| –Changed treatment due to AE or bleeding | 1 (1) | 0 | 1(1) | |
| Safetyb | AE | 147 (95.5) | 15 (93.8) | 69 (92.0) |
| Serious AE | 35 (22.7) | 5 (31.3) | 28 (37.3) | |
| Treatment-related AE | 82 (53.2) | 7 (43.8) | 29 (38.7) | |
| Treatment-related serious AE | 7 (4.5) | 1 (6.3) | 6 (8.0) |
| . | . | Romiplostim N = 157 . | SOC receiving rituximab N = 16 . | SOC N = 77 . |
|---|---|---|---|---|
| Baseline Characteristics | Age, median (range), years | 58 (18, 90) | 64.5 (29, 85) | 57 –86) |
| Female | 85 (54.1) | 10 (62.5) | 46 (59.7) | |
| ITP duration, median (range), years | 2.1 (0.02, 44.2) | 3.4 (0.01, 18.9) | 2.3 (0.01, 33.2) | |
| Platelet count (×109/L), mean (SD) | 30 (15) | 28 (20) | 25 (15) | |
| Disposition | Completed 52 weeks | 122 (78) | 7 (44) | 43 (56) |
| Discontinued due to: | 35 (22) | 9 (56) | 34 (44) | |
| –Didn't receive IP | 3 (2) | 0 | 2 (3) | |
| –Noncompliance | 1 (1) | 0 | 1 (1) | |
| –Adverse event | 7 (4) | 0 | 2 (3) | |
| –Withdrew consent | 4 (3) | 0 | 4 (5) | |
| –Subject request | 3 (2) | 0 | 0 | |
| –Alternative therapy | 6 (4) | 4 (25) | 14 (18) | |
| –Administrative decision | 1 (1) | 0 | 0 | |
| –Death | 1 (1) | 1 (6) | 1 (1) | |
| –Othera | 9 (6) | 4 (25) | 10 (13) | |
| Efficacy | Had splenectomy | 2 (1) | 5 (31) | 15 (20) |
| Had treatment failure, defined as ≥1 of the following | 6 (4) | 2 (13) | 10 (13) | |
| –Lack of efficacy | 2 (1) | 2 (13) | 4 (5) | |
| –Major bleeding | 3 (2) | 0 | 6 (8) | |
| –Changed treatment due to AE or bleeding | 1 (1) | 0 | 1(1) | |
| Safetyb | AE | 147 (95.5) | 15 (93.8) | 69 (92.0) |
| Serious AE | 35 (22.7) | 5 (31.3) | 28 (37.3) | |
| Treatment-related AE | 82 (53.2) | 7 (43.8) | 29 (38.7) | |
| Treatment-related serious AE | 7 (4.5) | 1 (6.3) | 6 (8.0) |
N (%) unless indicated otherwise.
Eg investigator decision, splenectomy, noncompliance, and lost to follow-up.
Safety based on population receiving IP, N = 154 romiplostim, N = 16 SOC-rituximab, N = 75 SOC.
Wasser:Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Off Label Use: Rituximab is not indicated for ITP. Boccia:Amgen: Equity Ownership, Speakers Bureau. Lyons:Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau. Mandanas:Amgen: Membership on an entity's Board of Directors or advisory committees. Pabinger:Glaxo: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Michel:Roche: Consultancy, Research Funding, Speakers Bureau; GSK: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau. Viallard:Amgen: Consultancy. Wang:Amgen: Employment, Equity Ownership. Lizambri:Amgen: Employment, Equity Ownership.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal