Abstract
Abstract 325
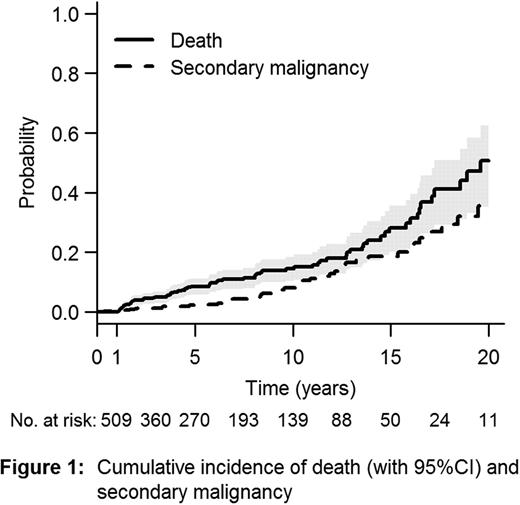
Fanconi anemia (FA) is a rare, genetically and phenotypically heterogeneous inherited disorder. The natural history of FA is characterized by progressive bone marrow failure (BMF) and an increased risk for development of malignancies. Allogeneic Hematopoietic Stem Cell Transplantation (HSCT) is considered the treatment of choice for FA patients with BMF or clonal evolution (acute myeloid leukemia or MDS). Most deaths related to HSCT occur within the first year after HSCT. Risk factors for the development of malignancies after HSCT are still incompletely defined in patients with FA. Our objectives were to evaluate risk factors for late mortality and secondary malignancies in 1-year survivors in the largest cohort of FA patients post-HSCT ever studied, so far. Patients and methods: Patients with FA reported to the European Blood and Marrow Transplant (EBMT) Group alive 1 year after a matched allogeneic HSCT were reviewed. Donor and recipient were matched if HLA A and B were identical at the generic level and HLA DRB1 at the allelic level. Cord blood as source of stem cells was excluded because of a few number of FA patients with very long-term follow-up (FU). Data was analyzed using proportional hazards and proportional cause-specific hazards models. Results: Between May 1972 and January 2009, 789 patients with FA who underwent first SCT were reported to the EBMT registry. 509 patients were alive 1 year post-HSCT and were included in the present study. 273 patients were male. Median age at HSCT was 9 years (range, 10 months to 44 years). The majority (77%) of patients had received stem cells from a related donor and bone marrow (80%) was the main source of stem cells. Irradiation was used as part of the conditioning regimen in 27% of the cohort, while fludarabine-based regimen was used in 29%. T-cell depletion (ex vivo and in vivo) was used in 41%. In January 2010, 15% (n=74) of the patients had died. Median age at death was 19 years. With a median FU of 6 years (1 to 28 years), the probability for survival after HSCT was 49% at 20 years (95%CI 38–65). The main causes of death were secondary malignancies in 52% of cases and treatment related mortality in 21%. Solid tumor represented 89% of the secondary malignancies. Cumulative incidence of death and secondary cancer are presented in Figure 1. A worse survival was observed in patients transplanted before year 2000 (Hazard ratio - HR: 2.24; 95%CI 1.06–4.71; p=0.034), in those transplanted because of clonal evolution (acute myeloid leukemia or MDS) (HR: 3.88; 95%CI 2.03–7.41; p<0.0001), in patients older than 10 years at SCT (HR: 2.00; 95%CI 1.26–3.18; p<0.004), and in patients transplanted more than a year after FA diagnosis (HR: 1.98; 95%CI 1.10–3.54; p=0.02). Without taking into account transplant period, HSCT after the age of 10 (HR 1.88 [1.17 to 3.03], P=0.009), clonal evolution before HSCT (HR 3.31 [1.72 to 6.39], P=0.0004) and previous chronic GVHD (HR 2.72 [1.65 to 4.46], P<0.0001) were associated with decreased survival. After adjustment for these factors, patients transplanted before 2000 still showed a worse survival (HR 2.09 [0.99 to 4.41], P=0.052). Using occurrence of a secondary malignancy as a time-dependent covariate, the hazard of death after this event was extremely high (HR 17.3 [9.70 to 30.7], P<0.0001). Independent risk factors for secondary malignancies included HSCT after the age of 10 (HR 2.89 [1.53 to 5.45], P=0.001), peripheral blood as source of stem cells (HR 3.06 [1.18 to 5.45], P=0.001) and previous chronic GVHD (HR 2.89 [1.53 to 5.45], P=0.001). Irradiation in the conditioning regimen and donor type (related versus unrelated) did not correlate with outcomes (both late survival and secondary malignancies). Conclusion: We found improved outcomes for patients with FA post-HSCT in recent years (>2000). However, long-term survival in FA patients after HSCT is still mainly affected by secondary malignancies (89% of solid tumors). Patients should be transplanted before the age of 10 with bone marrow as source of stem cells to try to avoid this complication. Moreover, chronic GvHD still emerges as a major cause for both secondary malignancies and mortality. Clearly improved method for prevention, early diagnosis and treatment of this complication are urgently needed. This study also highlights the need for very long-term FU for FA patients after HSCT.
Peffault de Latour:Alexion: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal