Abstract
Abstract 3183
The introduction of iron chelation treatment has led to a significant improvement in morbidity and overall survival in patients with transfusion-dependent anemias. Deferasirox is a once-daily, oral iron chelator approved for the treatment of transfusional iron overload in both adult and pediatric patients. The efficacy and safety of deferasirox in a variety of transfusion-dependent anemias has been established in numerous Phase II/III clinical trials. Since most patients with transfusion-dependent anemias require lifelong iron chelation therapy, there is a need to assess the long-term safety of deferasirox in both adult and pediatric patients.
To assess the safety profile of deferasirox in patients with transfusional iron overload in a real-world clinical setting. To further investigate the safety profile of deferasirox in patients with congenital erythrocyte disorders and transfusional iron overload, with ferritin levels <4000 ng/ml and without severe cardiac siderosis.
Between July 2009 and September 2010, 85 patients with transfusion-induced iron overload treated with deferasirox as per the approved product labeling were enrolled in the study. These data represent the 24-week planned interim analysis of a 12-month observational study on deferasirox safety profile in the treatment of pediatric and adult patients with transfusion-dependent anemias who were newly-treated with deferasirox. Safety was evaluated through the monitoring and recording of all adverse events and serious adverse events, as well as routine laboratory testing, including hematology, blood chemistry and hepatic function assessments.
The population had a median age of 37.6 years (range: 5.3–61.4) and a female to male ratio of 1.3. Beta-thalassemia (67.1%) was the most common transfusion-dependent anemia, followed by thalassemia intermedia requiring periodic transfusions (20.0%) and sickle cell anemia (12.9%). Mean baseline ferritin levels were 1502.1±870.5 (pediatric group: 1480.2±522.8 and adult group: 1503.6±891.4), while 53 out of the 85 patients (62.4%) had serum ferritin level above 1000 ng/ml. Mean baseline liver T2* value was 10.4±9.7 ms; 44.4% of patients demonstrated minimal liver iron deposition (MRI T2* > 6.3 ms), 51.4% had mild to moderate liver iron overload (T2* ≤ 6.3 ms), and 4.2% had severe liver iron overload (T2*<1.4 ms).
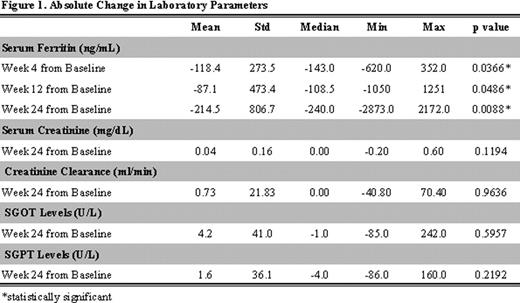
54 (63.5%) of patients analysed had been pre-treated with iron chelators and 31 (36.5%) were chelation-naïve. The initial average daily dose of deferasirox was 25.9±4.8 mg/kg, and 70.6% of patients had no dose modification during the 24-week follow-up period. A statistical significant decrease in median serum ferritin levels was observed by Week 24 (mean absolute change from baseline:-214.5 ng/mL; p=0.009) [Figure 1]. No statistically significant changes were observed in creatitine levels, creatinine clearance and transaminases by Week 24 [Figure 1].
37 ADRs were reported by 17 patients (20%) over the 24-week period. Among the most frequently observed ADRs (>5%) were epigastralgia reported by 7.1% of patients (6/85) and loose stools/diarrhoea by 5.9% of patients (5/85). The majority of ADRs reported (nevents=25; 67.6%) were graded as mild in severity, while 21.6% (nevents=8) were graded as moderate and 10.8% (nevents=4) as severe. Most ADRs (nevents=31; 83.8%) resulted in full recovery by Week 24. The overall incidence of SADRs was as low as 1.2% (in particular one patient experienced severe epigastralgia and upper extremity pain which resulted in her withdrawal from the study after four months of treatment). The all-cause discontinuation rate was 9.4% (8/85), while only two patients (2.4%) discontinued the study therapy due to ADR; 1 patient due to increased transaminase levels and 1 patient due to the aforementioned SADR.
These data highlight the safety profile of deferasirox in both adult and pediatric patients; the regular monitoring of serum ferritin levels as well as other iron-overload parameters and transfusion requirements play a major role in determining and optimizing the outcome of iron chelation therapy.
Ladis:Novartis Hellas S.A.C.I.: Investigator participating in a trial sponsored by Novartis. Drossou:Novartis Pharmaceuticals: Investigator participating in a trial sponsored by Novartis. Vini:Novartis Pharmaceuticals: Investigator participating in a trial sponsored by Novartis. Athanasiou-Metaxa:Novartis Hellas S.A.C.I.: Research Funding. Oikonomou:Novartis Hellas S.A.C.I.: Investigator participating in a trial sponsored by Novartis. Vlachaki:Novartis Hellas S.A.C.I.: Investigator participating in a trial sponsored by Novartis. Tigka:Novartis Hellas S.A.C.I.: Employment. Tzavelas:Novartis Hellas S.A.C.I.: Employment. Liakopoulou:Novartis Hellas S.A.C.I.: Investigator participating in a trial sponsored by Novartis. Adamopoulos:Novartis Hellas S.A.C.I.: Investigator participating in a trial sponsored by Novartis. Kattamis:Novartis Hellas S.A.C.I.: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal