Abstract
Abstract 3167
The membrane of erythrocytes is composed of a bilayer of phospholipids and cholesterol. It is strengthened by a membraneskeleton consisting of the proteins spectrin, ankyrin, pallidin, band 3 and band 4.1. Hereditary elliptocytosis (HE) is caused by mutations in the spectrin protein, resulting in a typical elliptocytic shape. These cells have a decreased deformability and a shortened lifespan. Most mutations in HE are located in the head to head self association site of the α- and β dimers of spectrin.
HE Patients with heterozygous mutations in α spectrin show little or no hemolysis because α spectrin is synthesized in an excess relative to β spectrin. In the heterozygous situation, plenty of normal wild type α spectrine (Wt α) is available for incorporation in to the membraneskeleton. In Hereditary Pyropoikilocytosis (HPP) with its bizarre shapes, a second mutation in the α spectrin is present, in addition to the HE mutation. It is responsible for a defect in the synthesis of α spectrin, resulting in the production of 50% functional α spectrin. This mutation is called LELY (Low Expression LYon). Thus, when the LELY mutation is located in trans on the allele in respect to the HE allele, the expression of the Wt α spectrin protein is reduced. As a consequence, more abnormal HE spectrin will be incorporated in to the membraneskeleton. This enhanced expression of the HE mutation results in an unstable membraneskeleton of the HPP cell.
The deformability of erythrocytes is measured using the ektacytometer LORRCA Maxsis of Mechatronics (Hoorn, The Netherlands). DNA was isolated from white blood cells from peripheral blood. The HE mutations are found by a PCR of the α spectrin exons where most mutations exists for HE, followed by DNA sequencing using the ABI prism genetic analyzer from Applied Biosystems. The LELY mutation is proven by a PCR of exon 40 of the α spectrin gene followed by RFLP agarose gel electrophoresis.(59 G/A) mutation and the LELY mutation are present. In addition to these Exon 2 and LELY mutations, a third mutation in Exon 6 (103 T/C) is found in the α spectrin of brother E.α spectrin is synthesized and incorporated into the membraneskeleton. The same mutations hold for brother E., but he has HPP. This is attributed to a third additional mutation in Exon 6 of the α spectrin gene. This Exon 6 mutation is located in trans to the Exon 2 mutation that in turn is in cis with the LELY mutation. Like in brother W. the expression of the Exon 2 mutated spectrin is reduced due to the LELY mutation. However relatively more of the Exon 6 mutated α spectrin comes available for the dimerization with β-spectrin resulting in an unstable membrane skeleton, causing the HPP of brother E.
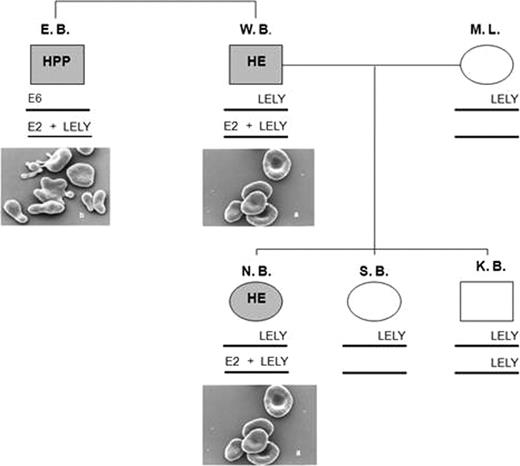
The B. family is examined for the presence of HE or HPP (figure 1). Brother E. has clinical symptoms, poikilocytes and a typical ektacytometric deformability matching HPP. Brother W. and his daughter N. have no clinical symptoms but elliptocytes and an ektacytometric deformability typical for HE. In all affected individuals the same Exon 2.
Presence of three mutations in α spectrin within the B. family, leading to Hereditary Elliptocytosis (HE) and Hereditary Pyropoikilocytosis (HPP). It concerns the HE mutations in Exon 2 (59 G/A) and Exon 6 (103 T/C). In addition, the LELY (Low Expression Lyon) mutation is present, which leads to reduced expression/synthesis of α spectrin.
Presence of three mutations in α spectrin within the B. family, leading to Hereditary Elliptocytosis (HE) and Hereditary Pyropoikilocytosis (HPP). It concerns the HE mutations in Exon 2 (59 G/A) and Exon 6 (103 T/C). In addition, the LELY (Low Expression Lyon) mutation is present, which leads to reduced expression/synthesis of α spectrin.
The combination of Exon 2 and LELY mutations normally leads to HPP. Concerning brother W. and his daughter N. this is not the case. They have a mild form of HE. The explanation for this finding is, that the Exon 2- and LELY mutation are located in a cis rather than in a trans position on the α spectrin gene (figure 1). In that case the mutated Exon 2 is less expressed and more Wt.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal