Abstract
Abstract 3157
Lenalidomide maintenance following induction with melphalan, prednisone, and lenalidomide has been shown to provide sustained disease control by significantly decreasing the relative risk of progression in NDMM patients [Palumbo, 2010]. HRQoL analyses, alongside efficacy and safety considerations, may help establish a more complete picture of an overall treatment profile. Initial findings on HRQOL have shown a well-balanced profile of MPR-R in terms of efficacy, safety and tolerability, with HRQoL improving both during MPR induction and lenalidomide maintenance [Dimopoulos, 2011]. Alternative findings on novel NDMM treatment have shown efficacy of melphalan, prednisone and bortezomib (VMP) treatment to be associated with an intermittent deterioration in patients' HRQoL [Dhawan, 2009].
This analysis considers the impact on HRQoL of ending treatment due to progressive disease (PD) or discontinuation due to other reasons (DC) for both MPR-R and MP patients. HRQoL scores were assessed for six domains, pre-selected based on clinical relevance: Global QoL, Physical Functioning, Fatigue and Pain (from EORTC QLQ-C30), and Disease Symptoms and Side Effects of Treatment (from EORTC MY20). Mean HRQoL scores measured at PD/DC, PD alone and DC alone were compared to mean HRQoL scores at baseline. In a second analysis, HRQoL observations during treatment (excluding baseline) were compared to HRQoL at end of treatment, analyzing observations for patients experiencing PD/DC, PD and DC via a mixed effects model that adjusted for time of observation as a fixed effect and patient as a random effect.
With a May 2010 follow-up, mean HRQoL at PD/DC had significantly improved from baseline for 3 of 6 domains with MPR-R - Global QoL (55.9 vs. 46.8; p=.021), Pain (30.4 vs. 42.6; p=.044) and Disease Symptoms (21.6 vs. 28.6; p=.031)(a) with non-significant changes in the other 3 domains. When assessing MPR-R patients experiencing PD, Global QoL (48.0 vs. 37.3; p=.029) and Pain (38.9 vs. 54.6; p=.046) also significantly improved from baseline, with no significant changes in the other 4 scales. MPR-R patients with DC experienced one significant improvement for Disease Symptoms (16.0 vs. 26.9; p=.007), with non-significant changes in the other domains. No significant HRQoL changes at end of treatment vs. baseline were observed for MP in any comparisons of the six domains considered.
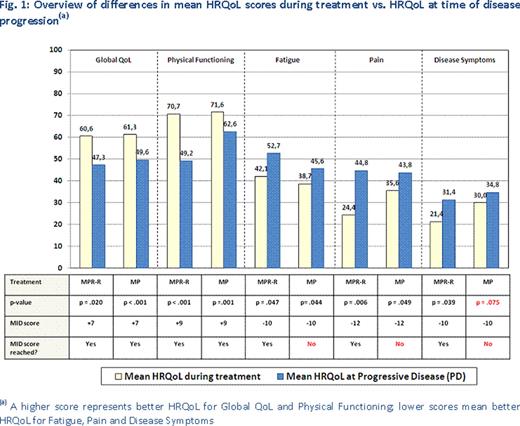
HRQoL during treatment was better in 33 of 36 comparisons when compared to HRQoL at end of treatment (assessment for six HRQoL domains; MPR-R and MP; PD/DC, PD and DC, i.e. 6×2×3), with significantly better HRQoL during treatment in 14 out of 36 observations. PD/DC significantly reduced HRQoL in terms of Physical Functioning (56.9 vs. 67.5, p =.003) and Fatigue (46.4 vs. 37.8, p=.026) for MPR-R and Global QoL (48.7 vs. 59.4, p <.001), Physical Functioning (61.6 vs. 70.3, p =.001) and Fatigue (46.7 vs. 39.3, p =.021) for MP. All significant differences involved PD, with no significant differences observed for DC. 9 of 12 (=6×2) HRQoL comparisons (see Figure 1) showed significantly higher HRQoL during treatment vs. PD; 2 of 3 non-significant HRQoL differences related to Side Effects of Treatment. HRQoL was clinically meaningfully higher during treatment than at progression in all 5 statistically significant MPR-R comparisons, but only in two MP comparisons (Global QoL and Physical Functioning), as determined by Minimal Important Differences (MIDs) [Dimopoulos, 2011]. The results indicate that delaying progression through continued MPR-R treatment is of key importance in strengthening NDMM patients' HRQoL.
Continuous lenalidomide significantly prolongs progression-free survival in NDMM patients. The underlying analysis documented higher HRQoL especially during MPR-R treatment compared to HRQoL at disease progression. Patients continuing on lenalidomide treatment experienced an elevated level of HRQoL, often reporting higher HRQoL levels at the end of MPR-R treatment vs. baseline. These findings further highlight the favourable balance between efficacy, tolerability and HRQoL with MPR-R.
Dimopoulos:Celgene: Consultancy, Honoraria. Off Label Use: Lenalidomide in newly diagnosed multiple myeloma. Delforge:Celgene: Consultancy, Honoraria, Speakers Bureau; Janssen-Cilag: Consultancy, Honoraria, Speakers Bureau. Hajek:Celgene: Honoraria; Janssen-Cilag: Honoraria; Merck: Honoraria. Petrucci:Celgene: Honoraria. Lewis:Celgene: Employment, Equity Ownership. Millar:Celgene: Consultancy. Zhang:Celgene: Employment, Equity Ownership. Mei:Celgene: Employment, Equity Ownership. Palumbo:Celgene: Consultancy, Honoraria; Janssen-Cilag: Consultancy, Honoraria; Ortho-Biotech: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal