Abstract
Abstract 3044
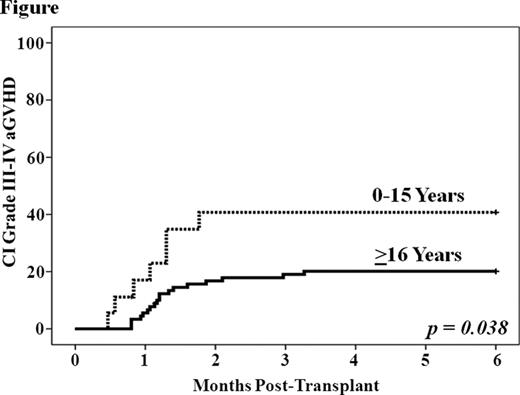
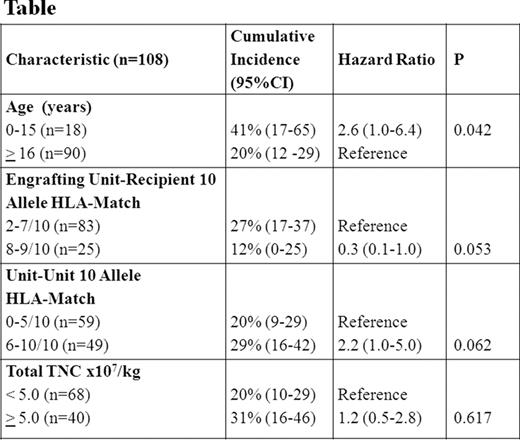
While the GVHD incidence after unrelated donor CBT is lower than expected for the degree of human leukocyte antigen (HLA)-mismatch, GVHD can be a serious complication and at our center has been the second most common cause of transplant-related mortality after DCBT. However, relatively little is known about DCBT GVHD manifestations, treatment response, and risk factors. Therefore, we evaluated 108 DCBT recipients (median 37 years, range 0.9–69) transplanted for hematologic malignancies. The majority had acute leukemia and high-risk disease. Patients received either myeloablative (n = 81) or non-myeloablative (n = 27) conditioning and 4–6/6 HLA-matched grafts. GVHD prophylaxis consisted of a calcineurin-inhibitor with mycophenolate mofetil, and no patient received anti-thymocyte globulin (ATG). With a median follow-up of 28 months (range 9–64), the cumulative incidences of day 180 grade II-IV and III-IV acute GVHD (aGVHD) were 52% (95%CI :42–62) and 24% (95%CI :15–32), respectively. The median onset was 40 days (range 14–161); the gut was most commonly affected (43/54, 80%) followed by skin (35/54, 65%). Twenty-five patients with mainly grade II gut aGVHD were treated with budesonide alone, 26 patients with predominantly grade III-IV aGVHD received systemic corticosteroids, and complete or partial treatment response was achieved in over 80% by day 56 of therapy. However, 41 patients had active GVHD after day 100 with the majority (25/41, 61%) having aGVHD (persistent, recurrent or late onset), particularly of the gut. Overlap syndrome and classical chronic GVHD were uncommon. Only 1 patient had oral ulceration, and no patient had moderate or severe ocular or sclerotic skin involvement, joint, or pulmonary GVHD manifestations. Univariate analysis of the association between patient/ graft characteristics and grade III-IV aGVHD showed the only significant factor associated with a higher severe aGVHD incidence was age 0–15 years (Figure). Diagnosis, patient ancestry, cytomegalovirus seropositivity, conditioning intensity, and infused cell doses/kg (total graft and engrafting-unit nucleated cell, CD34+ and CD3+) were not significant. A higher engrafting unit-recipient HLA-match of 8–9/10 was associated with a lower incidence of severe aGVHD, and a better unit-unit HLA-match of 6–10/10 was associated with a higher incidence of severe aGVHD, although these differences were not significant (p = 0.128 and 0.266, respectively). To further investigate these findings multivariate Cox regression analysis was performed (Table). Younger age was independently associated with a higher incidence of severe aGVHD (p = 0.042) whereas better engrafting unit-recipient match at 8–9/10 HLA-alleles was protective (p = 0.053). There was a trend toward better unit-unit HLA-match being associated with a higher incidence of grade III-IV aGVHD, but, surprisingly, total infused TNC/kg had no relationship. The 2-year PFS of 72% (95%CI :51–94) in children was higher than the 56% (95%CI :45–66) in adults despite their greater incidence of severe aGVHD. Nine patients (all adult) have died of GVHD including 5 patients initially treated with systemic corticosteroids and 4 with budesonide. We conclude that aGVHD after DCBT is common in the absence of ATG, predominantly affects the gut, and has a high rate of treatment response. Furthermore, GVHD after day 100 frequently has acute features. While the GVHD incidence does not preclude a high rate of survival, improved prophylaxis and treatment are needed. Notably, in contrast to single-unit CBT and adult hematopoietic stem cell transplantation, children receiving DCBT are at a higher risk for severe disease. A possible approach to reduce aGVHD in pediatric DCBT recipients with adequate CB units doses would be to prioritize high resolution HLA-match. Moreover, our data does not currently support an upper limit of infused TNC/kg in DCBT recipients. Further investigation of the biology underlying these unique observations (including the role of specific cellular subsets) should be a major priority.
Disclosures:
Off Label Use: Mycophenolate Mofetil as GvHD prophylaxis.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal