Abstract
Abstract 3038 FN2
FN2
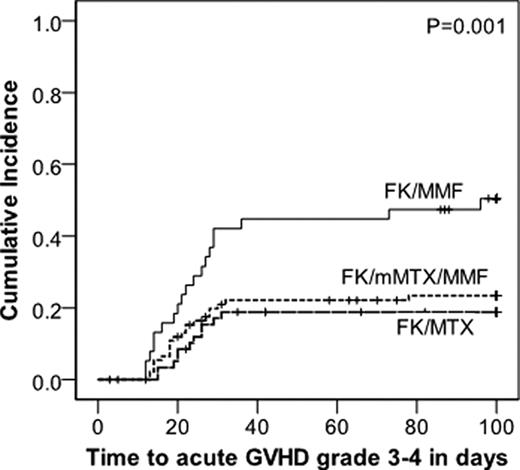
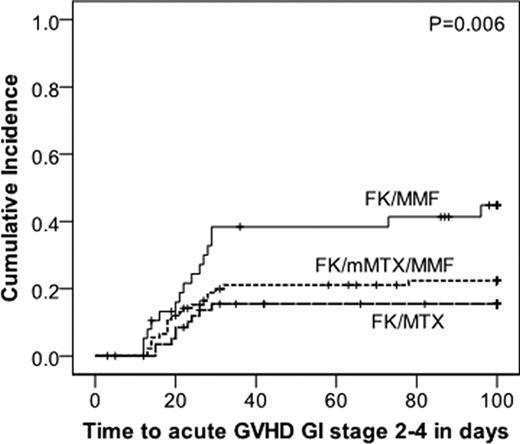
Methotrexate is a standard component of acute Graft-versus-Host Disease (aGvHD) prophylaxis although its use is limited by toxicity, especially mucositis. We compared 3 regimens utilizing tacrolimus (FK) ± methotrexate (MTX) ± mycophenolate (MMF) for aGvHD prophylaxis to determine the most effective and least toxic regimen. Patients were prospectively assigned to aGvHD prophylaxis with FK/μMTX/MMF, FK/MTX, or FK/MMF based on patient age, conditioning regimen and disease risk. For all 3 regimens, FK dosing was the same at 0.01 mg/kg IV BID with a 10% dose reduction every week starting at day 100 in the absence of aGvHD. MTX dosing was 10 mg/m2/d on days +1, 3, 6, & 11 (FK/MTX) or 2.5 mg/m2/day on days +1, 3, & 6 (FK/μMTX/MMF). MMF dosing was 1000 mg TID and discontinued on day 60 in the absence of aGvHD. Patients received allogeneic HCT conditioning with BU/CY, CY/TBI, or FLU/MEL. Acute GvHD stage/grade and toxicity data were collected prospectively. Patient characteristics are presented in the Table. 192 patients received FK/μMTX/MMF (n=94), FK/MTX (n=59), or FK/MMF (n=39). Patients who received FK/MTX were significantly younger, had lower risk disease, and more frequently received CY/TBI. FK/MMF resulted in a significantly higher incidence of grade 3–4 aGvHD (p=0.003) than either MTX-containing regimen (Figure 1) which was mainly due to a higher rate of GI involvement with FK/MMF (Figure 2, p=0.006). There was no difference in the rate of skin or liver aGvHD between the 3 prophylaxis groups. The incidence of grade 3–4 aGvHD between the FK/μMTX/MMF and FK/MTX groups was not significantly different. The incidence of grade 2–4 aGvHD between the 3 regimens was not significantly different, suggesting that MTX containing regimens prevent more severe grade 3–4 aGvHD. The incidence of grade 2–4 mucositis (requiring continuous narcotics or intubation for airway protection) occurred more frequently with FK/MTX (48%) than FK/MMF (18%, p=0.005) or FK/μMTX/MMF (21%, p=0.001). There was no significant difference in mucositis between FK/MMF and FK/μMTX/MMF. Sensitivity analyses by conditioning regimen intensity demonstrated similar results, except the difference in the rate of stomatitis which was no longer statistically significant. Overall (OS) and progression free survival (PFS) are better in the FK/MTX group, however, when stratified by age, the difference in OS and PFS between the 3 prophylaxis regimens was not significantly different. FK/μMTX/MMF (total 7.5 mg MTX/m2) is as effective as FK/MTX (total 40 mg MTX/m2) for aGvHD prophylaxis and both regimens are superior to FK/MMF. FK/μMTX/MMF results in less stomatitis, similar to FK/MMF, and both have significantly less toxicity than FK/MTX. A prospective phase 2 trial is planned to further define the clinical characteristics of FK/μMTX/MMF.
Table 1.
Patient characteristics by aGvHD prophylaxis regimen
| . | FK/μMTX/MMF N=94 N(%) . | FK/MTX N=59 N(%) . | FK/MMFN=39 N(%) . | p . |
|---|---|---|---|---|
| Age in years | <0.0001 | |||
| Median (range) | 52 (22–68) | 34 (19–63) | 54 (20–68) | |
| 19-39 | 13 (14) | 40 (68) | 3 (8) | |
| 40-59 | 60 (64) | 18 (31) | 30 (77) | |
| ≥60 | 21 (22) | 1 (2) | 6 (15) | |
| Diagnosis | 0.002 | |||
| ALL | 7 (7) | 15 (25) | 2 (5) | |
| AML | 39 (42) | 27 (46) | 22 (56) | |
| MDS | 12 (13) | 5 (8) | 6 (15) | |
| NHL | 15 (16) | 5 (8) | 8 (21) | |
| Other | 21 (22) | 7 (12) | 1 (3) | |
| CIBMTR risk category | 0.003 | |||
| High | 32 (34) | 7 (12) | 19 (49) | |
| Intermediate | 22 (23) | 14 (24) | 4 (10) | |
| Low | 36 (38) | 34 (58) | 16 (41) | |
| Non-malignancy | 4 (4) | 4 (7) | 0 (0) | |
| Hematopoietic cell source | NS | |||
| Bone marrow | 13 (14) | 11 (19) | 4 (10) | |
| Peripheral blood | 81 (86) | 48 (81) | 35 (90) | |
| Conditioning regimen | <0.0001 | |||
| BuCy | 12 (13) | 6 (10) | 5 (13) | |
| CyTBI | 4 (4) | 31 (53) | 2 (5) | |
| FluMel | 78 (83) | 22 (37) | 32 (82) | |
| Donor source | NS | |||
| Related | 33 (35) | 24 (41) | 19 (49) | |
| Unrelated | 61 (65) | 35 (59) | 20 (51) | |
| HLA match | NS | |||
| Match(10/10) | 76 (81) | 47 (80) | 32 (82) | |
| Mismatch(<10/10) | 18 (19) | 12 (20) | 7 (18) |
| . | FK/μMTX/MMF N=94 N(%) . | FK/MTX N=59 N(%) . | FK/MMFN=39 N(%) . | p . |
|---|---|---|---|---|
| Age in years | <0.0001 | |||
| Median (range) | 52 (22–68) | 34 (19–63) | 54 (20–68) | |
| 19-39 | 13 (14) | 40 (68) | 3 (8) | |
| 40-59 | 60 (64) | 18 (31) | 30 (77) | |
| ≥60 | 21 (22) | 1 (2) | 6 (15) | |
| Diagnosis | 0.002 | |||
| ALL | 7 (7) | 15 (25) | 2 (5) | |
| AML | 39 (42) | 27 (46) | 22 (56) | |
| MDS | 12 (13) | 5 (8) | 6 (15) | |
| NHL | 15 (16) | 5 (8) | 8 (21) | |
| Other | 21 (22) | 7 (12) | 1 (3) | |
| CIBMTR risk category | 0.003 | |||
| High | 32 (34) | 7 (12) | 19 (49) | |
| Intermediate | 22 (23) | 14 (24) | 4 (10) | |
| Low | 36 (38) | 34 (58) | 16 (41) | |
| Non-malignancy | 4 (4) | 4 (7) | 0 (0) | |
| Hematopoietic cell source | NS | |||
| Bone marrow | 13 (14) | 11 (19) | 4 (10) | |
| Peripheral blood | 81 (86) | 48 (81) | 35 (90) | |
| Conditioning regimen | <0.0001 | |||
| BuCy | 12 (13) | 6 (10) | 5 (13) | |
| CyTBI | 4 (4) | 31 (53) | 2 (5) | |
| FluMel | 78 (83) | 22 (37) | 32 (82) | |
| Donor source | NS | |||
| Related | 33 (35) | 24 (41) | 19 (49) | |
| Unrelated | 61 (65) | 35 (59) | 20 (51) | |
| HLA match | NS | |||
| Match(10/10) | 76 (81) | 47 (80) | 32 (82) | |
| Mismatch(<10/10) | 18 (19) | 12 (20) | 7 (18) |
*NS: P>0.1
* Other includes aplastic anemia, CLL, CML, Diamond Blackfan anemia, HL, MM, MPD, PLL.
Figure 1
Figure 2
Disclosures:
Hahn:Novartis:.
Author notes
*
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract
© 2011 by The American Society of Hematology
2011

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal