Abstract
Abstract 2996
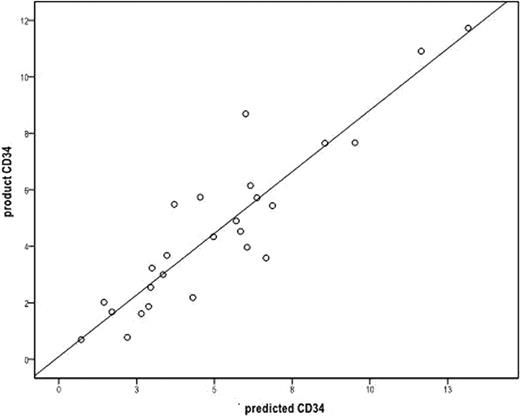
The final apheresis product CD34+ cell count per Kg of recipient weight is used to determine if additional apheresis procedures are necessary to collect the targeted amount of hematopoietic stem cells for autologous peripheral blood stem cell transplantation. Flow cytometric analysis of CD34+ cells can take several hours. A more timely technique to predict apheresis product CD34+ cell counts during apheresis may help determine if further administration of cytokines is necessary or if apheresis catheters can be removed, and overall improve the efficiency of patient (pt) care. We performed a retrospective review of all pts undergoing autologous peripheral blood stem cell mobilization with granulocyte-colony stimulating factor (G-CSF) alone, G-CSF and plerixafor, or with chemotherapy followed by G-CSF, from July 2010 through May 2011 who underwent peripheral blood stem cell collection with apheresis on the COBE Spectra cell separator. Linear regression models were used to formulate the calculation of pt blood volume based on the pre-apheresis CD34+ cell count per micro liter of blood, the final apheresis product CD34+ cell counts, and the amount of blood processed during the apheresis procedure. This calculated blood volume is expressed by the formula BV = 82.5(patients weight in Kg) + 793. We then prospectively evaluated the next consecutive pts who underwent stem cell mobilization and apheresis in June and July 2011. Twenty-seven apheresis collections were done on 26 pts. Fourteen pts were female, and 12 pts were male. Seventeen pts were diagnosed with myeloma, 6 pts with NHL, and 3 pts with other diseases. Twenty pts were mobilized with G-CSF (10 ug/kg daily) with apheresis to begin on day 5. Fifteen of the 20 pts required plerixafor on day 4 because of low peripheral blood CD34+ cell counts (< 10/ul). Six pts were mobilized with chemotherapy followed by G-CSF 10 ug/kg daily until peripheral CD34 cell counts recovered greater than 10/ul and then apheresis was started. Each pt had their blood volume calculated according to the formula above and the peripheral blood CD 34+ cell count was measured on the first and second day of apheresis. The peripheral blood CD 34+ cell count/ul was multiplied by 1000 and this product was multiplied by the calculated blood volume and then divided by the pts weight [(PBCD34+ cell count × 1000) × BV]/Kg to determine the predicted apheresis product CD34+ cell count, which was then compared to the actual apheresis product final CD34+ cell count. On the first day of collection the mean for the predicted product CD34+ cell count was 4.98 × 106 +/− 3.1 × 106, and the actual apheresis product CD34+ cell count was 4.61 × 106 +/− 2.90 × 106 (Pearson correlation r value of 0.913 and a p value <0.001)(see figure). Nineteen collections were evaluable on the second day of collection with the mean for the predicted product CD34 + cell count of 2.08 × 106 +/− 1.64 × 106, and the mean for the actual apheresis product CD34+ cell count of 2.29 × 106 +/− 0.768 × 106 (Pearson correlation r value of 0.620 with a p value of 0.005). There was no significant difference in the correlation between patients mobilized with G-CSF alone, G-CSF and plerixafor or after chemotherapy and G-CSF. In conclusion, a more accurate determination of patient blood volume allowed for a high degree of correlation on the first day of peripheral blood stem cell collection on the COBE Spectra machine between the predicted product CD34+ cell count and the actual apheresis product CD34+ cell count. An accurate prediction of the final apheresis product CD34+ cell count may allow for less cytokine administration, quicker removal of apheresis catheters, and more efficient disposition of patients undergoing peripheral blood stem cell collection.
Disclosures:
Shaughnessy:Otsuka: Honoraria, Speakers Bureau; Millenium: Honoraria, Speakers Bureau; Genzyme: Consultancy, Honoraria, Research Funding, Speakers Bureau.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal