Abstract
Abstract  295
295
Genetic events mediating transformation from the pre-malignant monoclonal gammopathies (MG) to myeloma (MM) are unknown. Previous FISH analyses have highlighted that most genetic lesions typical of MM are already present in MGUS. However, the genetic abnormalities characteristic of each evolving stage of MG have not been elucidated.
To obtain a comprehensive genomic profile of MG cases from the early to the late stages we performed for the first time high resolution analysis on purified plasma cells from 20 MGUS, 20 Smoldering MM (SMM) and 34 MM by high density 6.0 SNP-array. Ten matched non-tumor DNA samples were also included in the analysis. We examined DNA copy number alterations (CNA), copy number neutral loss of heterozygosity (CNN-LOH) and the spectrum of minimally altered regions which could contain relevant genes. Moreover, visual inspection allowed us to detect intermediate situations which corresponded to imbalances present in minor populations (less than 50%) coexisting with the major diploid population.
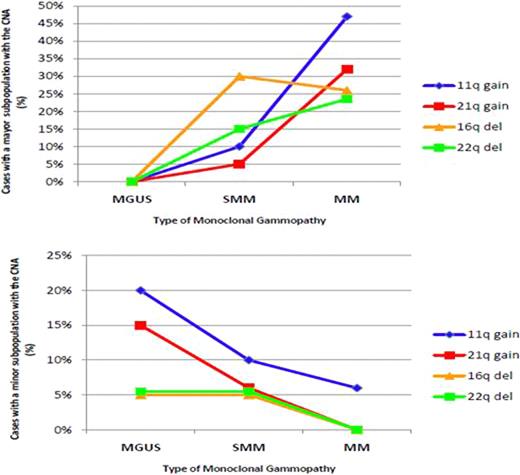
CNA were identified in 69 (93%) of the 74 patients analyzed with a median of 8 imbalances per abnormal case. The only 5 cases with no CNA corresponded to asymptomatic entities. We observed a progressive increase in the incidence of genomic imbalances from MGUS (median, 5/case) to SMM (media, 7.5/case) to MM (median, 12/case) (P=0.006; MGUS vs MM). In particular, gains on 1q, 3p, 6p, 9p, 11q, 19, 21q together with losses on 1p, 16q and 22q may be important genetic events associated with MGUS-MM transition, as they were less frequent in MGUS than in MM (P<0.038). Otherwise, 11p+ and 4q- would be implicated in the transition from SMM to symptomatic disease, as they were lower in SMM compared to MM (P<0.038). Chromosomal gains were usually associated with gains and losses with losses with the exception of 1q gains that were significantly associated with losses.
Overall, a total of 65 CNN-LOH were detected and 7 locus had a uniparental trisomy with a median number of genes of 188 per CNN-LOH. The frequency of CNN-LOH was higher in active MM as compared to the asymptomatic entities (MM, 53% vs SMM, 25% vs MGUS 25%; p=0.047). Most of the cases showed more than one region affected by this phenomenom. Of note, there were two identical interstitial CNN-LOH in one MGUS and one MM at 8q11.21-q11.23.
A strong association between genetic lesions and fragile sites (FRA) was also detected as more than one third of the focal-recurrent CNA and HZD, and more than a half of the minimal common regions and interstitial CNN-LOH overlapped with known FRA.
In summary, our study shows an evolving cytogenetic profile of increasing complexity from MGUS to SMM and to MM. However, although MM display more CNA and CNN-LOH than early steps, MGUS are as genetically aberrant as MM, and the transition from MGUS to MM is not associated to a particular chromosomal imbalance but rather to an expansion of altered clones already present in MGUS. The analysis of sequential samples from the same individual evolving from MGUS and SMM to active MM is essential to confirm these results. In addition, our study reveals new chromosomal regions involved in CNA, HZD and CNN-LOH. A comprehensive investigation of the genes contained in these regions may provide new insights in MM pathogenesis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal