Abstract
Abstract  2942
2942
Pomalidomide (pom) is a distinct immunomodulatory (IMid) drug with high response rates in relapsed MM, including patients previously treated with thalidomide, bortezomib and lenalidomide. However, its duration of response and long term outcome is unknown. We evaluated the long term outcomes of the first Mayo Clinic cohort of pts treated with Pom.
Pts with relapsed/refractory myeloma with 1–3 prior therapies were enrolled. Pom 2 mg/d was given orally continuously on 28 day cycle with weekly dexamethasone 40 mg. All pts received DVT prophylaxis with aspirin, heparin or warfarin.
60 pts with relapsed MM were enrolled from Nov 2007-Aug 2008. Median age was 65.5; 36 were male. Median time from diagnosis was 45.6 mo (9.–-192.5). Nineteen (32%) were high-risk according to mSMART (msmart.org), and 78% were ISS Stage 2/3. Prior therapies included transplant (65%), bortezomib (33%), thalidomide (47%), lenalidomide (35%) [previous IMiD 60%] and radiation (38%). Toxicities at least possibly attributed to Pom included G3/4 anemia (5/0%), leukopenia (17/3%) and thrombocytopenia (3/0%). G3/4/5 non-hematological toxicities occurred in 30 (50%) and included fatigue (18/0/0%), pneumonia (8/2/2%), hyperglycemia (5/0/0%) and constipation (5/0/0%). Only one pt had grade 3 neuropathy. Treatment adjustment for occurred in 30 pts (50%) for pom (mostly for neutropenia) and 35 pts (58%) for dex. A median of 11.5 cycles were administered.
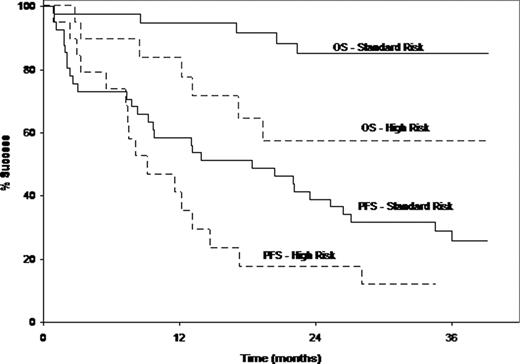
With median follow up of 30.1 months (mo), overall response was seen in 39 (65%) [4 sCR, 5 CR, 16 VGPR, 14 PR]. Median time to first reponse (≥PR) was 1.7 mo (0.8–14.8). Median duration of response (in responders of ≥PR) was 21.3 mo. Median PFS was 13 mo and the 2 year OS rate was 76%. Standard risk pts had median PFS of 18.4 mo and 2 year OS rate was 85%; high risk pts had median PFS of 9.2 mo and 2 year OS rate was 57%. (Figure 1)
Pom/dex is highly effective and well tolerated in relapsed MM, with a response rate of 65% even in pts with multiple prior therapies, including other IMiDs, bortezomib and transplant. The response is also durable, with median duration in excess of 21 months. Pom/dex provides long term benefit with median PFS 13 months and 2 year OS rate of 76%.
Kumar:Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees. Stewart:Celgene Corporation: Consultancy, Research Funding; Millenium: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Onyx: Consultancy, Research Funding. Fonseca:Consulting :Genzyme, Medtronic, BMS, Amgen, Otsuka, Celgene, Intellikine, Lilly Research Support: Cylene, Onyz, Celgene: Consultancy, Research Funding. Lacy:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal