Abstract
Abstract 2926
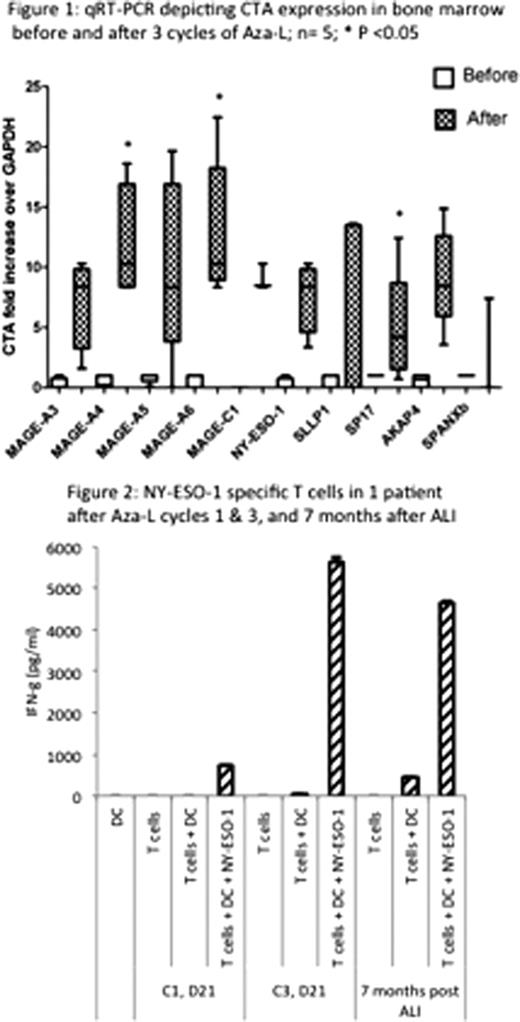
Patients with multiple myeloma (MM) undergoing high dose therapy and autologous stem cell transplantation (SCT) remain at risk for disease progression. Maintenance therapy may delay recurrence but is associated with toxicities, highlighting the need for alternative strategies for long-term disease control. Malignant plasma cells in MM patients occasionally express highly immunogenic cancer testis antigens (CTA). CTA expression is regulated by DNA methylation, and may be increased by 5-azacitidine (Celgene corp., Summit, NJ) (Aza), a DNA hypomethylating agent. The addition of lenalidomide (Celgene corp.) (L) may augment any ensuing adoptive CTA-specific immune response. These immune effector cells may then be collected and adoptively transferred in a setting of lympho-depletion and minimal residual disease following SCT, serving a maintenance function. To demonstrate the feasibility of this approach, we initiated a phase II clinical trial of Aza administered sequentially with L in patients with residual disease following initial therapy (NCT01050790). Three cycles of Aza (75 mg/m2 day 1–5 SQ) and L (10 mg PO daily, day 6–21) were administered every 4 weeks; autologous lymphocytes (AL) were collected around day 21 of the 2nd and 3rd cycles of Aza-L and cryopreserved. Subsequent stem cell mobilization was followed by melphalan 200 mg/m2 and SCT. GM-CSF was used post-transplant for hematopoietic engraftment. Autologous lymphocyte infusion (ALI) was performed between day +30 to +60.
Twelve patients have been enrolled; median age is 60 years (range 40–69). Eight are African American, 10 had disease in first partial remission (PR) and 2 in very good PR (VGPR) at the time of initiation of therapy. Median of 2 prior regimens had been administered (range 1–2) and 6 had prior therapy with L. Stage at diagnosis was II (n=6) and III (n=6) and 4 had abnormal cytogenetics. Eight patients have completed all three cycles of Aza-L; 2 developed grade 3 neutropenia, no other grade 3 to 4 toxicity has been observed. Eight patients have gone through both AL collections, 21 days following cycles 2 and 3 of Aza-L, yielding 0.90±0.41 and 0.81±0.36 ×108 CD3+ cells/kg (mean ± SD) with the first and second procedures. Three patients had further disease response, (1 near complete remission, 2 VGPR) and 5 had stable disease following three months of Aza-L, representing a median 18% decline in para-protein levels before and after therapy. So far 8 patients have undergone stem cell mobilization with either GCSF alone (n=6) or GCSF + plerixafor (n=2), and have collected 11.2±3.3 ×106 CD34+ cells/kg body weight. To date 6 patients have undergone SCT (tandem SCT in 1). Neutrophil engraftment was at median of 14 days (13–14), and no unexpected post transplant toxicities were observed. Four patients received ALI at a median 42 days following SCT with no immediate or remote infusional toxicities. With a median follow up of 9 months post-transplant, all four ALI recipients are progression free with either complete remission (n=1) or VGPR (n=3).
In conclusion, we demonstrate the safety and feasibility of epigenetic modification resulting in over-expression of antigenic targets in MM. This may then be exploited in formulating adaptive immunotherapy protocols in these patients. Adoptively transferred cells may maintain long-term surveillance against malignant plasma cells in patients with MM.
Toor:Celgene corporation: Research Funding. Off Label Use: azacitidine in multiple myeloma. Manjili:Celgene corporation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal