Abstract
Abstract 2855
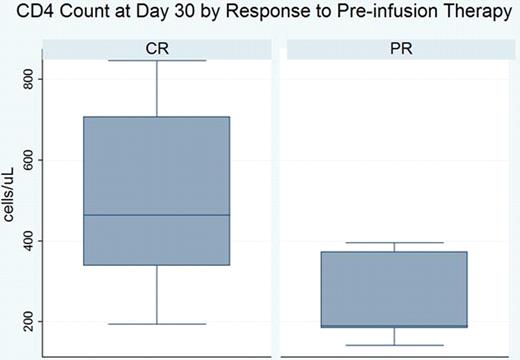
Fludarabine-based therapies prolong survival of patients (pts) with chronic lymphocytic leukemia (CLL), but deplete CD4 T-cells causing immunosuppression which increases the risk of infection and may limit control of minimal residual disease by the immune system. Adoptive immunotherapy using autologous CD3/CD28-costimulated T-cells expanded ex vivo (ACTC) enhances immune reconstitution after fludarabine-based therapy of lymphoma (Schuster et al Blood abstract 2007). Methods: We conducted a multicenter phase I/II trial of ACTC following fludarabine-based therapy of CLL. After leukapheresis, pts received fludarabine-based therapy. 8 – 12 weeks after last chemotherapy, responding pts (CR, PR) received a single dose of ACTC prepared from autologous T-cells collected before chemotherapy. Results: 34 pts are enrolled (median age 61; male 24, female 10). To date, 19 pts are evaluable for studies of immune reconstitution at ≥90 days after ACTC infusion. Prior to ACTC, 11 pts received FCR and 1 pt FR as first therapy, while 5 pts received FCR and 2 pts FCR + bevacizumab for previously treated CLL. Median ACTC dose was 6.8E+9 CD3 cells (range: 4.88E+07 −1.05E+10). After chemotherapy, before ACTC infusion (day 0), median CD4 and CD8 counts for all evaluable pts were 119 (range: 12–573) and 80 (range: 4–682), respectively; 30 days after ACTC (day 30), median CD4 and CD8 counts were 373 (range 141–846) and 208 (range: 25–879), respectively. The increases in CD4 and CD8 counts between days 0 and 30 were statistically significant (p = 0.0003 for both CD4 and CD8 cell counts) and remained significantly increased between days 0 and 90 for both CD4 (p = 0.0004) and CD8 (p = 0.005) cell counts. ACTC dose was not associated with change in CD4 or CD8 count. Pts in complete remission (CR) at the time of ACTC infusion had significantly higher CD4 counts on day 30 than pts in partial remission (PR) [median day 30 CD4 count for CR = 464 (range: 194–846) vs PR = 190 (range: 141–395), p = 0.02], but not on days 0 and 90. There was no difference in CD8 counts for pts in CR or PR on days 0, 30, and 90. There were no SAEs; 1 pt developed AIHA after ACTC. For 19 evaluable pts at a median follow-up of 12 months (range: 3.2–22), PFS from ACTC infusion is 80%. Conclusions: ACTC infusion results in a dose-independent acceleration of CD4 and CD8 T-cell recovery after fludarabine-based chemotherapy compared to historical controls. CD4 counts on day 30 are significantly higher for pts in CR at the time of ACTC infusion. Further studies of immune reconstitution and function following ACTC are in progress.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal