Abstract
Abstract  2841
2841
Genomic aberrations are widely used markers to predict survival of patients with chronic lymphocytic leukemia (CLL). Deletion of 13q as sole abnormality (del 13q) has been associated with a favorable prognosis. However, whether the clinical impact of monoallelic del 13q (mono-del 13q) is different as compared to biallelic del 13q (bi-del 13q), remains controversial. Similarly, it is unsettled, if there is an impact of clone size on outcome of patients with del 13q.
The prognostic impact of mono-del 13q and bi-del 13q, as well as the proportion of cells with del 13q prior therapy was studied in the CLL8 trial of the GCLLSG (1st line FC vs FCR). Pretherapeutic variables, response rates (ORR, CR), progression free survival (PFS), and overall survival (OS) were compared between the groups.
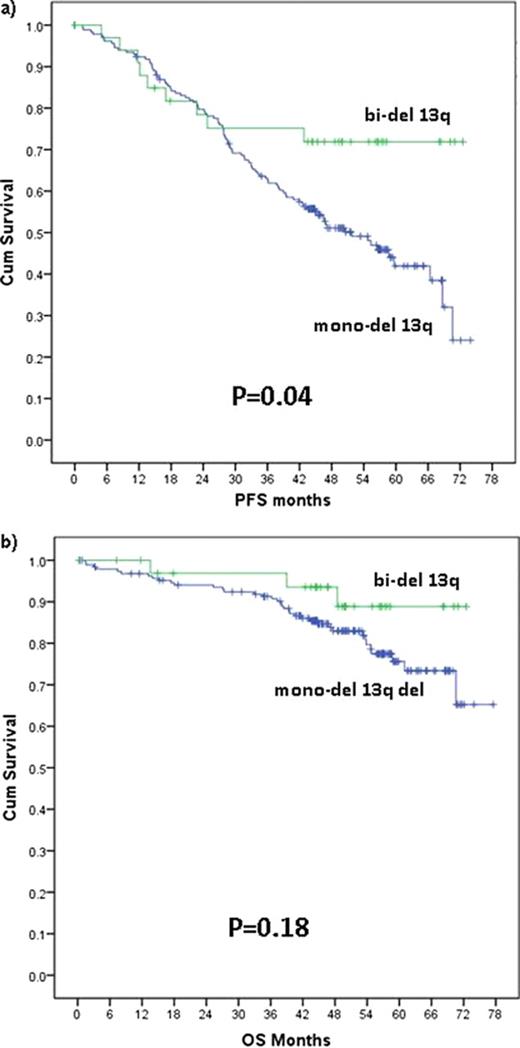
224 patients with del 13q as single aberration were analyzed. 189 patients (84%) had a mono-del 13q, and 35 (16%) a bi-del 13q. B-symptoms were observed more frequently in patients with bi-del 13q (63% vs. 38%, P<.01). No significant differences were seen when comparing all other baseline characteristics between the two groups (Binet/Rai stage, ECOG, leukocyte count, sex, levels of thymidine kinase and ß2-mikroglobuline, IGHV mutation status, usage of V3-21 genes and TP53 mutation) and there was no significant difference in ORR (87% vs 89%) and CR (34% vs 37%) rates. However, PFS was significantly longer in patients with bi-del 13q (median not reached vs. 52 months, P=.04; Fig. 1a). When treated with FCR, patients with bi-del 13q had significantly higher CR rates than those with mono-del 13q (60% vs 47%), while there was no significant difference when treated with FC (25% vs 22%). Similarly, FCR resulted in longer PFS in patients with bi-del 13q as compared to mono-del 13q patients (P=.06). Interestingly, this difference was not observed for FC therapy. Regarding OS, there was a trend towards better outcome in patients with bi-del 13q when combining both treatment arms (P=.18, Fig. 1b). Comparison of treatment arms regarding OS was limited due to few events. However, it was of note that not a single death was observed in the group with bi-del 13q when treated with FCR. Interestingly, among bi-del 13q cases, PFS and OS was independent of the IGHV mutation status (P=.78 for PFS, P=.42 for OS). In contrast, patients with mono-del 13q with mutated IGHV genes had a significantly longer PFS and OS than those with unmutated IGHV genes (P<.01 for PFS, P<.01 for OS).
Regarding the clone size of del 13q, there were no significant differences in response rates, PFS and OS in relation to the % of cells with del 13q. This was irrespective of using clone size as a continuous variable, using the median value as cut-point, or separating clone size into quartiles (percentile 25: 42%, median: 76%, percentile 75: 88% del 13q). Median clone sizes in patients achieving a CR, PR, SD or PD were 74% (N=77), 78% (N=119), 74% (N=14) and 70% (N=1), respectively. Median PFS in months was 66, 59, 57 and 39 in quartiles 1–4 (P=.44) and median OS was not reached in either quartile.
Although most baseline characteristics were not significantly different when comparing mono-del 13q and bi-del 13q, the presence of a bi-del 13q was associated with significantly longer PFS and a trend towards longer OS after FC-based 1st line treatment in CLL. Bi-del 13q appeared to predict for higher CR rates and longer PFS with FCR as compared to FC treatment. For patients with bi-del 13q, PFS and OS were independent of IGHV mutation status. The size of the aberrant del 13q clone did not impact response rate, PFS or OS.
Fischer:Hoffmann La Roche:. Hallek:Hoffmann-la Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Stilgenbauer:Hoffmann La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Travel Grants.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal