Abstract
Abstract 2836
Monoclonal antibodies (mAb) are an effective but non-curative treatment of CLL. Although the mechanisms of action of mAb in vivo are not fully defined, complement dependent cytotoxicity (CDC) appears to play an important role. We have previously shown that addition of ofatumumab (OFA) significantly increases alemtuzumab (ALM) CDC in vitro and identified a subpopulation of CLL cells that are intrinsically resistant to activated complement. We propose that resistance to CDC could be an important cause of mAb treatment failure in CLL patients. To test the hypothesis that CLL cells treated in vivo with OFA would be resistant to subsequent OFA retreatment in vitro, we studied CLL cells and serum from 10 previously untreated patients with progressive CLL treated with pentostatin, cyclophosphamide, and ofatumumab (PCO).
Patients received 300 mg of OFA followed by 2 mg/m2 of pentostatin and 600 mg/m2 of cyclophosphamide on day 1 of therapy. Samples were taken prior to treatment (S1), immediately after the OFA infusion and prior to chemotherapy (S2), and post-chemotherapy before the second dose of OFA on day 2 (S3). Mononuclear blood cells isolated from EDTA anticoagulated blood by Ficoll-Paque centrifugation were purified to achieve a concentration of > 90% CLL. CDC was assayed at a concentration of 2 × 106/ml CLL cells with 10 mg/ml of ALM (Genzyme), OFA (G.S.K.) or rituximab (RTX)(Genentech) in AIM V medium (Invitrogen, CA) and 10% normal human serum (10%NHS)(Sigma, MO) as a source of complement for 1 hour at 37°C. Absolute viable cell counts were measured by flow cytometry using counting beads (Trucount, BD, CA) and propidium iodide staining (Sigma, MO) with a FACSCalibur (BD, CA) and CellQuest Pro software (BD, CA). Percent CDC was calculated relative to counts for CLL cells treated with only 10%NHS. Binding of mAb and C3b to CLL cells was measured by flow cytometry with mouse anti-human Fc antibody FITC-HB43 and anti-C3b antibody FITC-7C12. C5 deficient serum (C5-serum)(Sigma, MO) was used to examine mAb and C3b binding without CDC. Results were expressed as delta mean fluorescent intensity (dMFI) relative to cells treated without mAb in 10%NHS or C5-serum as appropriate. Complement (CH50) was measured based on lysis of opsonized sheep red cells using standard methods. OFA concentrations in serum samples were determined by measuring the level of binding to Daudi cells compared to standards as previously described.
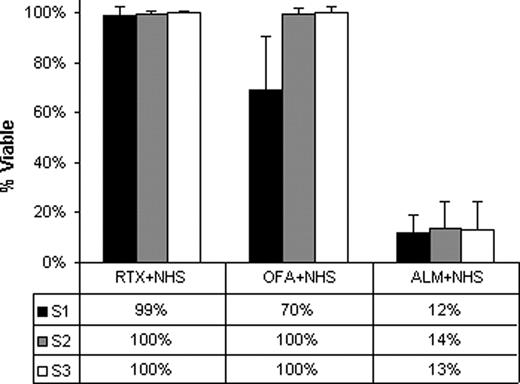
S1 CLL cells were significantly more susceptible to in vitro OFA CDC (median 30%) than S2 and S3 cells (both 0%, p=0.002)(Fig. 1). In contrast, high levels of CDC (median 86 – 88%) were induced in vitro by ALM in all these samples and prior in vivo exposure to OFA did not affect susceptibility to ALM CDC in vitro (p>0.1). CLL cells from S2&S3 had low levels of OFA binding both prior to and post in vitro exposure to OFA (median dMFI 2 and 3 respectively) which was significantly less than OFA binding for S1 (median dMFI 38 p=0.002) likely reflecting in vivo trogocytosis of bound OFA and CD20, as previously described for RTX. In vitro ALM binding was higher than OFA (median dMFI>150) in all specimens. C3b binding was low in S2&S3 both before and after in vitro OFA exposure (median dMFI 3 and 4 respectively) with significantly higher levels in S1 (median dMFI 94, p=0.002). C3b binding was higher in all CLL cells treated with ALM (median dMFI>358). OFA treatment resulted in marked decreases in serum complement levels in S2 (median 86%, range 65–98%) and S3 (median 78% range 61–88%) compared to S1. The median serum OFA concentration was 18.5 μg/ml (range 7.3–50.4) in S2 and 19.8 μg/ml (range 0–32.3) in S3.
Circulating CLL cells from patients treated with OFA are resistant to in vitro OFA CDC primarily because of low levels of expression of CD20. The reduction in complement titers could limit in vivo CDC but we found no evidence that low levels of OFA were important. Cells surviving OFA in vivo do retain sensitivity to ALM CDC. Our data support the previous description of trogocytosis in CLL patients treated with RTX and suggest that lower doses of these mAb, that promote far lower levels of trogocytosis, could be more effective in sustaining CDC. In addition, our study provides pre-clinical data to support a clinical trial of combination therapy with OFA and ALM for CLL.
This study was supported by funding from GlaxoSmithKline and the University of Iowa/Mayo Clinic Lymphoma SPORE (CA097274).
Taylor:Genmab: Consultancy; Glaxo Smith Kline: Research Funding. Zent:GlaxoSmithKline: Research Funding; Genentech: Research Funding; Genzyme: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal