Abstract
Abstract  2831
2831
Prognosis and need of treatment in CLL is currently determined by clinical staging systems of Binet and Rai. Recent research has focused on prognostic factors that may predict a poor prognosis independent of the clinical stage. Markers which have shown independent prognostic information are serum parameters and genetic factors (genomic aberrations, IgHV and p53 mutational status). To investigate the relevance of these different factors, we performed a pooled analysis using the data of three multicenter German CLL Study Group phase III trials (CLL1, CLL4 and CLL8). Based on this analysis we propose a prognostic score for previously untreated patients with early and advanced CLL.
Patients were recruited between 1997 and 2006 into three phase III trials: 715 in CLL1 (“watch and wait” versus fludarabine (F)), 362 in CLL4 (F versus F and cyclophosphamide (FC)) and 817 patients in the CLL8 trial (FC versus FC and rituximab (FCR)). Serum parameters and genetic factors were centrally analyzed prior to treatment. The main end point of all statistical analyses was overall survival. First, univariate analyses were performed including variables of different groups such as baseline characteristics, stage of disease, laboratory results, molecular cytogenetics, mutational status and serum parameters. Next, multivariate Cox regressions were applied including all parameters that showed a significant association with overall survival in univariate analyses. To create a prognostic score we developed a weighted grading algorithm for independent factors based on ranges of hazard ratios. Finally, a prognostic score was defined as the sum of single ratings of adverse factors. According to this score, four different risk groups for overall survival could be identified.
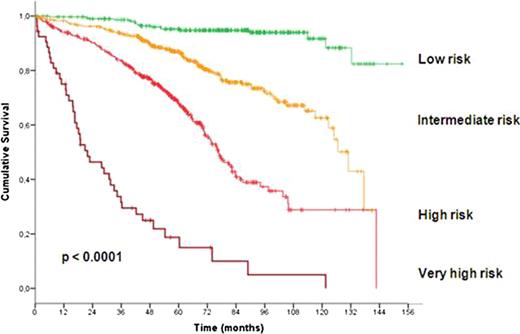
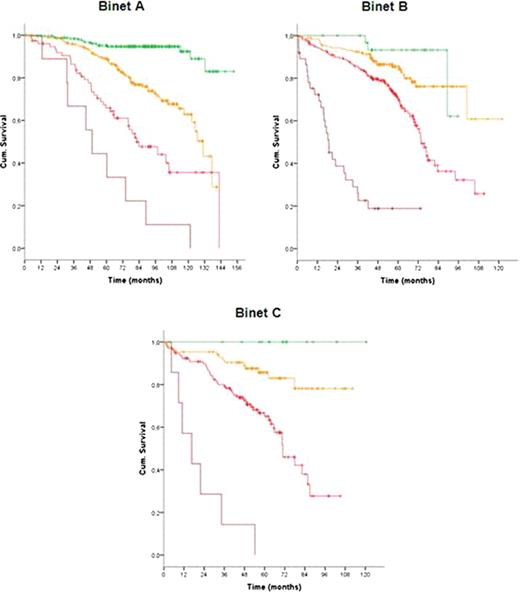
In total 1948 patients were eligible for the pooled analysis with a median age of 60 years (range, 30 to 81 years). After a median observation time of 63.4 months 485 deaths were reported. At study entry, 799 patients (42.4%) were at Binet stage A, 717 (38.0%) at Binet stage B and 370 (19.6%) at Binet stage C. Almost all considered variables were significantly associated with outcome and therefore included in the multivariate analysis. Based on the data of 1223 patients for whom all parameters were available, multivariate Cox regressions were performed and identified gender, age, ECOG score, del(17p), del(11q), IgHV mutational status, serum β2-microglobulin and serum thymidine kinase as independent factors for overall survival. Deletion 17p was the strongest adverse factor. Neither the clinical staging (Rai, Binet) nor the treatment modality were independent prognostic factors for overall survival. Similarly, the time interval between first diagnosis and study entry was not an independent prognostic factor. Due to the great differences between hazard ratios of independent factors, we developed a weighted grading system based on a simple algorithm to assign an individual grade to each adverse factor. By using this weighted grading, four different prognostic groups could be separated: low risk (score 0 – 2), intermediate risk (score 3 – 5), high risk (score 6 – 10) and very high risk (score 11 – 14) (figure 1). Overall survival rates were significantly different for these four groups with 95.2%, 86.9%, 67.7% and 18.7% survival after 5 years for the low, intermediate, high and very high risk group, respectively (p<0.0001). Moreover, within the group of patients showing a deletion 17p the score could distinguish patients of a high risk and a very high risk group (p<0.0001). Finally, the score could predict the individual risk for short overall survival independent of and within the different Binet or Rai stages (p<0.0001) (figure 2).
While Binet and Rai staging systems may remain important for the initial clinical assessment due to their simplicity, our prognostic score using a weighted combination of genetic and serum markers is superior to predict the overall survival of CLL patients.
Survival curves according to risk groups as defined by prognostic score
Pflug:Hoffmann-la Roche: Travel grant; Mundipharma: Travel grant. Eichhorst:Hoffmann La Roche: Honoraria, Research Funding, Travel Grants; Mundipharma: Research Funding, Travel Grants; Gilead: Consultancy. Bergmann:Celgene: Honoraria. Döhner:Hoffmann-la Roche: Research Funding. Stilgenbauer:Hoffmann La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Travel Grants. Fischer:Hoffmann La Roche: Travel Grants. Hallek:Hoffmann-la Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal