Abstract
Abstract 2742
The treatment of chronic myeloid leukemia (CML) was revolutionized by the introduction of tyrosine kinase inhibitors (TKIs) to the clinical practice. However, there is still a significant group of patients who due to drug resistance do not fully benefit from the therapy. Among various reasons of drug resistance, the changes of intracellular concentration of TKIs caused by the activity of drug transporters may have profound effect on clinical activity of the drug. Intracellular concentration of imatinib is a result of: drug influx into the cell and drug efflux out of the cell. The latter is mediated by ATP-binding casette (ABC) transporters - MDR/ABCB1 and BCRP/ABCG2. According to the recent knowledge modulation of membrane cholesterol level may change the activity of ABC transporters by changing their conformation. Hence we decided to evaluate potential effect of statins, widely used hypocholesterolemic drugs, on antileukemic activity of imatinib in cell lines transformed with BCR/ABL oncoprotein and primary human CML CD34+ cells.
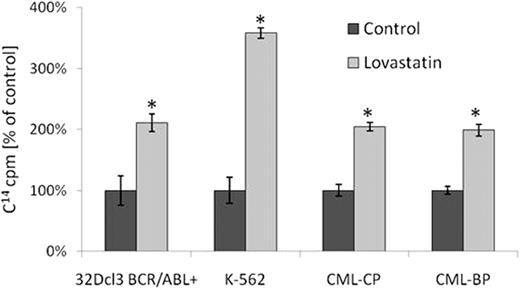
In a set of experiments involving cytotoxic assays (i.e. clonogenic assay, XTT, trypan blue exclusion assay) we revealed that lovastatin, a member of statin family, is able to enhance imatinib cytotoxicity both in established cell lines (32Dcl3 BCR/ABL+, K562) as well as in primary CML CD34+ cells obtained from patients in various stages of the disease, including those clinically resistant to imatinib with no detectable ABL kinase domain mutations. In the same time, lovastatin alone and in combination with imatinib did not influence BCR/ABL negative cells (32Dcl3 wild type, HL-60, CD34+ cells obtained from healthy blood donors). As measured using specific, fluorescent-labeled substrates for ABC transporters (rhodamine-123 and Bodipy-FL-prazosin respectively), lovastatin significantly inhibits MDR/ABCB1- and BCRP/ABCG2-mediated efflux capacity. Similar effects were observed for other statins. The addition of cholesterol to the samples previously incubated with lovastatin completely reversed inhibitory activity of statins. To confirm these results we examined direct changes of intracellular concentration of imatinib itself using radiolabeled 14C-imatinib. In these settings, statins caused 2–3-fold increase in intracellular imatinib concentration in murine and human cell lines (32Dcl3 BCR/ABL+, K562) and also in primary CML CD34+ cells including clones resistant to imatinib (Figure 1). Statins did not influence initial concentration of imatinib what may suggest that their effects on imatinib concentration are not mediated by changes in OCT-1 activity. In addition, statins did not influence expression of MDR/ABCB1 and BCRP/ABCG2 drug transporters measured using qPCR nor ABC proteins levels measured using Wester blotting. Combination of imatinib and lovastatin induced cell cycle arrest and increased percentage of apoptotic leukemic cells measured using flow cytometric analysis after propidium iodide staining. Lovastatin also reduced imatinib-induced phosphorylation of the adaptor protein CrkL.
Statins increase intracellular concentration of imatinib in BCR/ABL-positive cells.
Statins increase intracellular concentration of imatinib in BCR/ABL-positive cells.
CML-CP, CML-BP–CD34+ cells obtained from CML patient in chronic phase or blast crisis respectively; *p<0,05
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal