Abstract
Abstract 2725
Sezary syndrome (SS) is an aggressive leukemic form of cutaneous T cell lymphoma (CTCL) and is generally considered incurable. Until now no true animal model for SS exists which could be used for the screening of novel compounds against the disease. We successfully developed a bioluminescent xenograft mouse model for SS to noninvasively monitor tumor cell engraftment and progression and to measure the effects of treatments on tumor burden.
A fusion protein was constructed consisting of the monomeric mutant red fluorescent mCherry, and the synthetic-firefly Luciferase by cloning the mCherry gene into the plasmid vector pGL4.13 [luc2/SV40] (Promega) carrying the luciferase gene, thus obtaining the pGLCherryLuciferase plasmid, where the Cherry and the luciferase genes formed one open reading frame. Analysis of pGLCherryluciferase transfected human embryonic kidney 293 (HEK 293) cells via flow cytometry and luciferase activity confirmed that the cherryluciferase fusion protein retained its dual bioluminescent/fluorescent activity in vitro. The H9 cell line derived from a SS patient was transfected with this plasmid using the transfection reagent, Effectene. The transfected H9 cells were injected subcutaneously into the flank of 5–7 week old female severe combined immunodeficiency (SCID-Beige) and NOD/Shi-scid/IL-2Rγnull (NOG) mice (Taconic Laboratories, Germantown, NY). After confirmation of a bioluminescent signal, NOG mice were assigned to the vehicle control group or treatment groups, which received romidepsin (1.2 or 2.3mg/kg on day1, 4, 8 and 11, ip) or pralatrexate (30mg/kg on day1, 4, 8 and 11, ip). In vivo bioluminescence imaging over 3 weeks was performed using an IVIS imaging system (Caliper Life Sciences, Alameda, CA). Mice were anesthetized with 3% isoflurane and imaged for 1 minute following ip injection of 150 mg/kg of D-luciferin. For quantification of light intensities in time course experiments, an equal constant analysis gate was defined for all mice and individual tumor photon counts were determined using Living Image®software (Caliper Life Sciences, Alameda, CA).
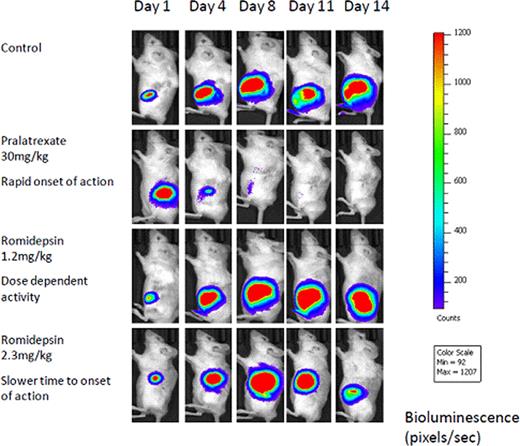
The SCID/Beige mice did not permit any engraftment of the H9 injected cells whereas all the NOG mice exhibited tumor growth and progression. This suggests that the NOG mice are superior animal recipients for xenotransplantation of Sezary cells, potentially making them a preclinical tool to understand tumorigenesis and drugs effects in this rare malignancy. All mice bearing H9-mCherry-luc cells developed a bioluminescent signal at the site of inoculation within 7 days of injection of transfected cells. Sequential quantitative signals from bioluminescent imaging over the 3-week period were significantly lower in the mice treated with pralatexate compared with the control group (Figure 1). The mice treated with 1.2mg/kg of romidepsin had no effect on tumor suppression whereas the mice that received the higher dose of romidepsin demonstrated decrease of tumor growth suggesting dose-dependent tumor inhibition. Mice treated with pralatrexate demonstrated diminished bioluminescent signal as early as after the first dose compared to the mice treated with higher dose of romidepsin which produced a slower time to onset of activity. No mice treated with pralatrexate or with the higher dose of romidepsin exhibited toxicity.
This novel bioluminescent xenograft mouse model of SS enables non-invasive, sensitive, quantitative evaluation of disease progression in living animals and evaluation of pharmacologic factors in real time. We are able to detect and monitor lymphoma cell growth before the presentation of clinical manifestations. Further this model recapitulates our understanding of behavior of drugs used in the treatment of lymphomas such as pralatrexate which has a rapid onset of action compared to romidepsin that has a delayed time to onset of activity. This represents the first bioluminescent animal model of human CTCL that is intended to be used to investigate novel treatment platforms in preclinical studies. This preclinical model also compliments the ongoing phase 2 trial of pralatrexate in relapsed or refractory CTCL. Further in vivo studies to evaluate synergy of promising new agents in this novel mouse model of SS have begun and will be reported.
O'Connor:Celgene: Consultancy, Research Funding; Merck: Research Funding; Novartis: Research Funding; Spectrum: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal