Abstract
Abstract 2680
Romidepsin is a class 1 selective histone deacetylase (HDAC) inhibitor approved in the United States for patients with relapsed/refractory cutaneous and peripheral T-cell lymphomas. Potential cardiac class effects of HDAC inhibitors have been variably reported. We have previously reported that detailed electrocardiogram (ECG) analyses from 110 patients across 3 clinical studies showed clinically insignificant, transient ECG changes that were not associated with functional cardiovascular changes. The primary objective of the study described herein was to further evaluate the potential of romidepsin to prolong QT and assess any possible relationship between romidepsin plasma concentration and heart rate-corrected QT interval duration (QTc).
Patients from GPI-06–0005, an exploratory phase 1 study of adult patients with advanced malignancies treated with 1-hour or 4-hour intravenous (IV) romidepsin infusion, received ECG assessments on Day 1 of cycles 1 and 2: baseline (both pre- and post-antiemetic premedication) and at each pharmacokinetic (PK) sampling time (0.25, 0.5, 1, 2, 3, 4, 6, 8, and 24 hours after initiation of romidepsin dosing). Patients either received a 4-hour IV infusion at 14 mg/m2 on Days 1, 8, and 15 of each 28-day cycle for 6 cycles or for 1 cycle followed by 8–12 mg/m2 infused over 1 hour for 5 cycles. Post-antiemetic baseline was included to isolate the effects of romidepsin as antiemetics alone may increase the QTc interval. All ECGs were read by a central ECG laboratory and overread by a cardiologist for verification of interval measurements. Standard analyses as defined in the International Conference on Harmonization E14 Guidance, Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs were used. Nonlinear mixed effect modeling was used to analyze the exposure-response relationship of romidepsin and QTc.
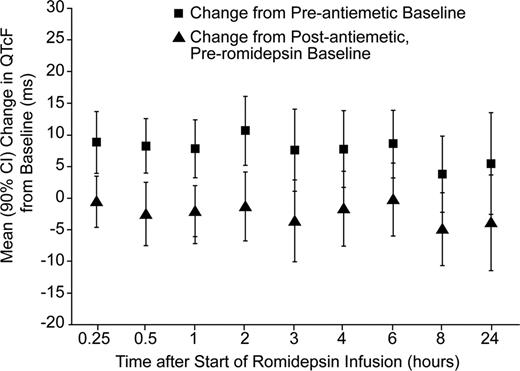
ECG data were available for 26 patients who received romidepsin at 14 mg/m2 over a 4-hour IV infusion (approved dosing regimen). Fourteen of these patients also received 8–12 mg/m2 romidepsin over a 1-hour IV infusion; 3 patients received 8 mg/m2, 6 received 10 mg/m2, and 5 received 12 mg/m2. The majority of the 26 patients were female (62%) and White (89%); median age was 61.7 years (range, 44–82 years). Patients who received romidepsin at 12 mg/m2 as a 1-hour infusion had a median plasma Cmax up to 2.5-fold higher than that observed with the approved dosing regimen. The time courses of mean QTcF (Fridericia correction), QTcI (individual correction) and ΔQTcF, as well as median romidepsin plasma concentration were examined. No marked trend was evident, except for some evidence of a modest change in QTcF that was temporally dissociated from the pharmacokinetic profile. However, no consistent pattern of pharmacodynamic counterclockwise hysteresis was noted across or within patients. Exposure-response modeling demonstrated no statistically significant effect of romidepsin concentration on QTc. As shown in the Figure comparing change in QTcF relative to baseline pre-antiemetic administration or post-antiemetic administration, there was a slight mean decrease from post-antiemetic baseline in QTcF at each time point following romidepsin administration, and a mean increase relative to pre-antiemetic baseline. Mean changes in QTcF from the post-antiemetic, pre-romidepsin baseline for the 14 mg/m2 4-hour infusion were negative for all assessments. The maximum upper bound of the 90% confidence interval (CI) for the change from post-antiemetic, pre-romidepsin baseline was 5.59 ms noted at the 6-hour time point; mean change in QTcF at this time was –0.11 ms. No patients in this study developed a QTcF > 450 ms in the 24-hour period following the start of infusion. No trends were observed across lower dose levels (8-, 10-, and 12-mg/m2) for the 1-hour romidepsin infusions.
No concentration-dependent effects of romidepsin on the duration of QTc interval were identified, including at romidepsin exposures up to more than 2.5-fold higher than the approved and clinically used dosing of 14 mg/m2 as a 4-hour IV infusion.
Mean Change (90% CI) in QTcF from Baseline over Time Following Dosing of Romidepsin 14 mg/m2 IV over 4 Hours
Mean Change (90% CI) in QTcF from Baseline over Time Following Dosing of Romidepsin 14 mg/m2 IV over 4 Hours
Godfrey:Geron Corporation: Consultancy; Neurocrine Biosciences: Consultancy; Agios: Consultancy; Adnexus: Consultancy; Lundbeck, Inc.: Consultancy; Vertex Pharmaceuticals: Equity Ownership; Anoixis Corporation: Employment; Celgene: Consultancy. Off Label Use: Dosing in studies is off label. Cabell:Celgene: Consultancy. Balser:Celgene: Contracted Consultancy. Wolfson:Celgene: Contracted Consultancy. Nichols:Celgene: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal