Abstract
Abstract 268
Obinutuzumab (GA101) is the only type II glycoengineered, humanized anti-CD20 monoclonal antibody in clinical development for the treatment of lymphoma and chronic lymphocytic leukemia. The efficacy of GA101 monotherapy in patients with relapsed/refractory indolent non-Hodgkin's lymphoma (iNHL) is under investigation, and here we report response and long-term results from a Phase I/II study (BO20999).
Phase I was a non-randomized dose-escalating study (3 + 3 design; n = 21) with GA101 monotherapy (50–2,000 mg) on Days 1 and 8 of Cycle 1, and Day 1 of Cycles 2–8 (21-day cycles). The primary objective was to determine the safety and pharmacokinetics of GA101 in patients with NHL. In Phase II patients with iNHL were randomized to receive GA101 at one of two doses: 1,600 mg on Days 1 and 8 of Cycle 1 and then 800 mg on Day 1 of Cycles 2–8 (1,600/800 mg; n = 22), or 400 mg on Days 1 and 8 of Cycle 1 and on Day 1 of Cycles 2–8 (400/400 mg; n = 18). The primary efficacy endpoint was end of treatment response, assessed 4 weeks after the last infusion. Secondary endpoints included safety, pharmacokinetics, best overall response (BOR), and progression-free survival (PFS).
Patient baseline characteristics (Phase II)
| Characteristic . | 400/400 mg (n = 18) . | 1,600/800 mg (n = 22) . | All (n = 40) . |
|---|---|---|---|
| Histology, n | |||
| Follicular | 14 | 20 | 34 |
| Other | 4 | 2 | 6 |
| Clinical stage at diagnosis (Ann Arbor) | |||
| I–II | 0 | 3 | 3 |
| III–IV | 17 | 18 | 35 |
| Unknown | 1 | 1 | 2 |
| Median age, years (range) | 51.0 (42–79) | 61.5 (44–76) | 60.5 (42–79) |
| Median number of prior treatments (range) | 3 (1–8) | 3 (1–11) | 3 (1–11) |
| Prior stem cell transplant, n | 6 | 8 | 14 |
| Previous rituximab, n | 17 | 21 | 38 |
| Rituximab refractory*, n | 12 | 10 | 22 |
| Characteristic . | 400/400 mg (n = 18) . | 1,600/800 mg (n = 22) . | All (n = 40) . |
|---|---|---|---|
| Histology, n | |||
| Follicular | 14 | 20 | 34 |
| Other | 4 | 2 | 6 |
| Clinical stage at diagnosis (Ann Arbor) | |||
| I–II | 0 | 3 | 3 |
| III–IV | 17 | 18 | 35 |
| Unknown | 1 | 1 | 2 |
| Median age, years (range) | 51.0 (42–79) | 61.5 (44–76) | 60.5 (42–79) |
| Median number of prior treatments (range) | 3 (1–8) | 3 (1–11) | 3 (1–11) |
| Prior stem cell transplant, n | 6 | 8 | 14 |
| Previous rituximab, n | 17 | 21 | 38 |
| Rituximab refractory*, n | 12 | 10 | 22 |
Rituximab refractory: those patients who have had no response or a response of <6 months to a rituximab-containing regimen (rituximab monotherapy or in combination with chemotherapy) at any point during their treatment history.
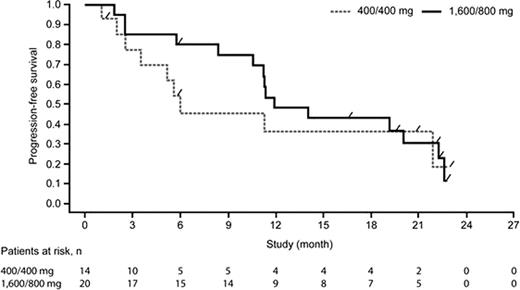
Progression-free survival (Phase II, follicular non-Hodgkin's lymphoma)
Progression-free survival (Phase II, follicular non-Hodgkin's lymphoma)
In conclusion, GA101 monotherapy shows encouraging efficacy with a higher response observed at a higher dose (1,600/800 mg vs 400/400 mg). Phase III trials are ongoing in GA101 in combination with chemotherapy for first-line treatment of patients with advanced iNHL.
Salles:Roche: Consultancy, Honoraria. Morschhauser:Roche: Honoraria; Celgene: Consultancy, Honoraria. Wenger:Hoffmann La Roche: Employment. Wassner-Fritsch:Roche: Employment. Asikanius:Roche: Employment. Cartron:Roche: Consultancy, Honoraria; GSK: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal