Abstract
Abstract 2669
The survival of adult BL has improved with intensification of multi-agent chemotherapy, although 2-year survival rates remain <65–70%. Efforts to improve survival, as well as decrease treatment-related toxicities are needed. Further, there are no prospective clinical studies to date that have examined the addition of rituximab into the CODOX-M/IVAC regimen.
Eligible patients for this investigator-initiated, 5-site phase II clinical trial included: newly diagnosed BL and B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and BL (according to WHO 2008 definition) regardless of HIV status. Eligibility for HIV+ patients included: no evidence of multi-drug resistant HIV infection or concurrent AIDS defining illness and CD4 count >350/mcL. Patients were classified as low risk (LR) if they had all of the following factors present: 1) normal LDH, 2) stage I/II disease, 3) ECOG performance status (PS) <2, and 4) no mass >10 cm. All other patients were “high risk” (HR). LR patients received 3 consecutive cycles of CODOX-M, while HR patients received 4 alternating cycles of CODOX-M and IVAC. For CODOX-M, methotrexate 3.0 gram/m2 i.v. was used. Further, liposomal doxorubicin (40 mg/m2) was utilized in lieu of doxorubicin (day 1 of all CODOX-M cycles), while intravenous rituximab (500 mg/m2) was added to days 0 and day 8 of each CODOX-M cycle and days 0 and 6 of IVAC cycles. In addition, as a corollary analysis, frequent assessment of ejection fraction (EF) was performed in all patients (baseline, s/p 2 cycles, and 4 weeks after completion of therapy).
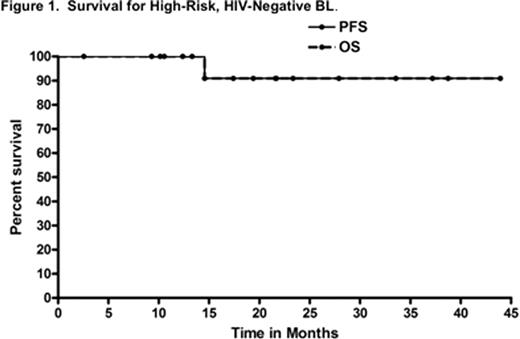
Twenty-five patients (22 male and 3 female) enrolled from March 2007 through April 2011. The median age was 44 years (range, 23–70 years). Furthermore, 5 (20%) patients were >60 years. All patients had classical BL, while 1 patient had concomitant BCL-2 expression. There were 20 HR and 5 LR patients; 3 of the HR and 1 LR patient were HIV+, while the remaining patients were HIV-negative. Median PS at study entry was 1, while PS=2 in 6 (24%) patients. Further, 3 (15%) HR patients had + central nervous system disease (2 parenchymal, 1 leptomeningeal). Additionally, 7 (35%) HR patients had bulky disease >10 cm (2 (10%) with dominant mass >20cm), 8 (40%) of all patients had bone marrow involvement, and 15 (75%) had an elevated LDH. 24 of 25 patients were evaluable for toxicity and response/survival. Therapy was completed at a median of 13.5 weeks (range, 11–20) for HR patients and a median of 10 weeks for LR (range, 9–12). With respect to toxicity, myelosuppression was overall comparable (58% of patients experienced grade 4 thrombocytopenia with only 4% grade 4 anemia) to prior CODOX-M/IVAC data, while the incidence of mucositis also appeared similar to prior reports (38% grade 3, 13% grade 4). Other clinically relevant grade 3 toxicities included neutropenic fever (33%), transaminitis (33%), diarrhea (8%), elevated creatinine (8%), and seizure (4%). Notably, there was no grade 3 or 4 neuropathy. After 2 cycles of therapy, two grade 2 and two grade 3 cardiac events were noted (all depressed EF, no clinical evidence of congestive heart failure). The two grade 3 events occurred in a 70-year-old and 69-year-old man, both with HR disease, and the latter with history of myocardial infarction. Among all patients, the median change in EF at baseline vs study end was: −2% (range, −22% to +11%). In terms of outcomes, the response rate after 2 cycles of therapy was 100% with a 67% complete remission (CR) rate. At a median follow-up of 24 months, the 2-year PFS and OS rates for all patients were 86% and 86%, respectively (LR 2-year PFS and OS both 100%; and HR 2-year PFS and OS both 82%). Furthermore, the 2-year PFS and OS rates for HR, HIV-negative patients were 91% and 91%, respectively (see Figure 1), while the disease-specific survival (DSS) for this subgroup of patients was 100%. Of the 3 deaths on trial, 2 were due to progressive disease in HIV+ HR patients, while the 3rd was a 71 year-old HIV-negative HR subject who died in CR at 14 months from unknown causes.
Altogether, the integration of rituximab and liposomal doxorubicin into CODOX-M/IVAC for adult BL was feasible and associated with similar tolerability compared with prior reports. Additionally, this regimen was associated with excellent survival rates, especially for HIV-negative BL.
Evens:Ortho-Biotec: Research Funding. Off Label Use: Doxil in the treatment of Burkitt's lymphoma. Carson:Genentech: Speakers Bureau. Nabhan:Genentech: Research Funding, Speakers Bureau. Gregory:Genentech: Advisory Board. Gordon:Genentech: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal