Abstract
Abstract 2618
Adoptive transfer of antigen-specific T cells emerged as an attractive strategy to provide cancer patients with immune cells of a desired specificity. The efficacy of such adoptive transfers has been demonstrated in clinical studies. However, enrichment and expansion of tumor-specific T cells are time-consuming and often ineffective, due to the low frequency of tumor-specific precursors in vivo. Alternatively, polyclonal T cells can be genetically modified with chimeric antigen receptors (CARs) to provide these cells with new antigen specificities. CARs consist of a binding moiety specifically recognizing a tumor cell surface antigen fused to a signaling chain derived from a lymphocyte activating receptor. The chimeric receptor approach is able to bypass many of the mechanisms by which tumors avoid immunorecognition, such as MHC down-regulation, lack of expression of costimulatory molecules, and induction of T cell suppression.
Acute myeloid leukemia (AML) is an intrinsically resistant disease and even though the majority of the patients initially respond to chemotherapy, the 3-year survival rate is still low. A promising target for immunotherapy of AML is CD33, which is absent on normal pluripotent hematopoietic stem cells and normal tissues, but is present on leukemic blasts in 85–90 % of adult and pediatric AML cases independent of the subtype of AML.
Human CAR-engrafted T cells mediate effector functions against CD33+ target cells.
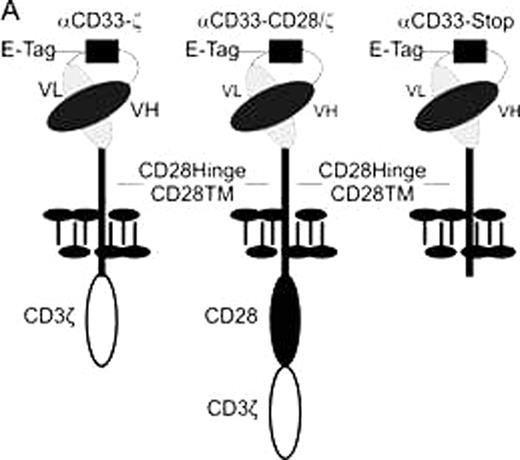
A. Schematic representation of the CD33-specific CARs. VL: variable light chain; VH: variable heavy chain; E-Tag: linker epitope in between the VL and the VH chain.
Human CAR-engrafted T cells mediate effector functions against CD33+ target cells.
A. Schematic representation of the CD33-specific CARs. VL: variable light chain; VH: variable heavy chain; E-Tag: linker epitope in between the VL and the VH chain.
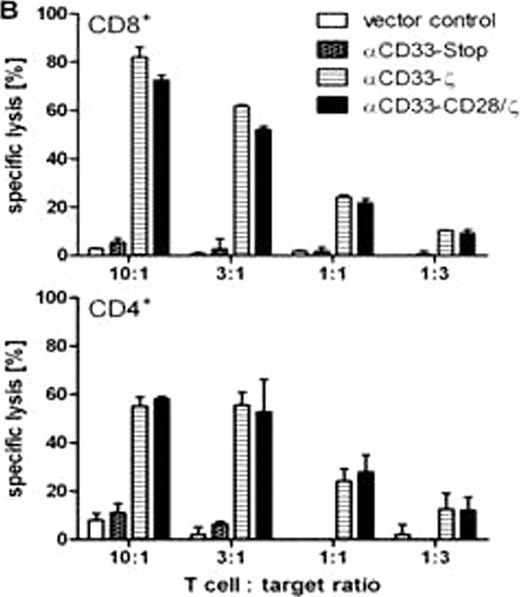
B. Cytotoxic effector functions of CAR engrafted human CD8+ and CD4+ T cells against the CD33+ blast line U937 were measured in a standard chromium release assay after 6h of co-incubation.
B. Cytotoxic effector functions of CAR engrafted human CD8+ and CD4+ T cells against the CD33+ blast line U937 were measured in a standard chromium release assay after 6h of co-incubation.
C. Killing of patient-derived AML blast by allogeneic CAR engrafted T cells was measured in a flow cytometer by exact cell count after 48h of co-cultivation. Three independent donor/patient pairings are shown.
C. Killing of patient-derived AML blast by allogeneic CAR engrafted T cells was measured in a flow cytometer by exact cell count after 48h of co-cultivation. Three independent donor/patient pairings are shown.
Until now, one major obstacle for an adoptive therapy of genetically modified T cells is the limited amount of T cells that can be isolated from AML patients and modified in vitro. For an efficient in vitro expansion restricted to CAR modified T cells from patients we developed a new method based on a novel CAR-mediated strategy. For this purpose, magnetic beads were coated with an antibody recognizing an epitope (Fig. 1A, E-Tag) which we included in the linker domain between the heavy and the light chain of the scFv portion of the CAR. Adding such magnetic beads to cultures of CAR modified T cells, the CAR engrafted T cells were expanded to similar extends as polyclonal T cell populations with anti-CD3/anti-CD28 coated beads. Furthermore, the antibody-coated beads can be used to isolate the CAR engrafted T cells after expansion and get rid of any contaminating non-modified cells. It may also be useful for elimination of CAR expressing T cells in vivo if necessary. The feasibility of the described method was not limited to CD33 specific CARs but was also functional for CARs equipped with scFvs of other specificities. Therefore, it might be universally applicable for the expansion and preparation of CAR modified T cell grafts in vitro before adoptive transfer back in patients.
Taken together, we describe novel humanized CD33-specific CARs that (I) can be specifically expanded, (II) specifically eliminated, if necessary, and (III) may therefore become a novel potent treatment option for a cellular therapy of AML patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal