Abstract
Abstract  2552
2552
Long-term survival rates for adults with acute lymphoblastic leukemia (ALL) remain poor at 40% when compared with pediatric ALL pts. This is partly attributed to the lack of predictive markers for risk stratification in adult ALL. Childhood ALL studies have shown that higher absolute lymphocyte count (ALC) during or following induction predicts longer event free survival (EFS). We hypothesized that adult ALL patients with higher ALC following induction therapy will have prolonged EFS and overall survival (OS).
We conducted a retrospective chart review of 230 adult pts (age 18–64 yrs) with de novo ALL diagnosed between 1993–2010 at the Cleveland Clinic and Stanford, 198 of whom were evaluable (112 Cleveland Clinic, 86 Stanford). Prior studies in the pediatric population identified an ALC of 350 cells/μL as a cutoff that was predictive of outcome; we assessed this cutoff as well as other ALC metrics including day 1, 3, 7, 11, 14, 17, 20, 25, and 28 counts, nadirs and area under the curves at various time points, and the rate of ALC decline. We also evaluated the impact of gender, age at diagnosis, cytogenetics (CG), WBC at diagnosis, and transplant. The Kaplan-Meier method was used to summarize OS and EFS and the log-rank test was used for univariable analysis. The Cox proportional hazards model, stratified by institution, was used for multivariable and univariable analyses of measured factors.
Median age: 38 yrs (range 18–64); Gender: 58% (114) male; CG risk: 62 (31%) poor, 46 (23%) miscellaneous, 54 (27%) normal, 36 (19%) no growth or not done; 178 (90%) B-cell lineage; median WBC at diagnosis 9.5 K/μL (range 0.5–760). Eighty-three percent of pts (165) achieved a CR with induction chemotherapy. The majority of pts (178, 90%) received a vincristine/prednisone/anthracycline-based induction regimen. Four pts (2%) received double-induction on trial S0333 (induction 1: vincristine/prednisone/anthracycline; induction 2: high dose cytarabine/mitoxantrone); eight pts (4%) hyperCVAD; and eight pts (4%) high dose cytarabine/mitoxantrone. Twenty-one pts (11%) received imatinib in combination with chemotherapy. Forty-six pts (23%) received an allogeneic transplant in CR1.
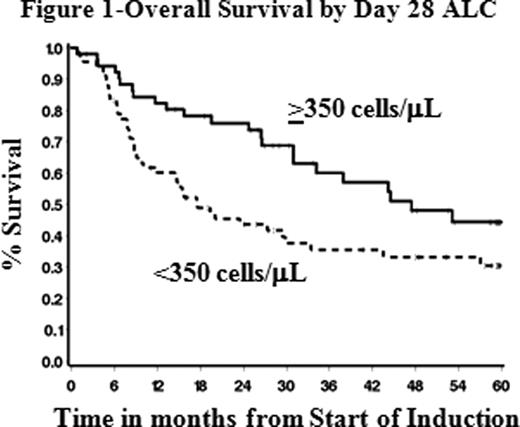
The median OS and EFS for this cohort were 29.5 and 24.9 mos, respectively. A number of the metrics were found to be associated with outcome; however we found that ALC at day 28 with a cut-point of <350 cells/μL was the strongest predictor of OS and EFS. The median ALC at Day 28 was 300 cells/μL (range 0–4000 cells/μL). Patients with ALC ≥350 cells/μL had a median OS of 47.4 mos compared to 17.6 mos for patients with ALC <350 cells/μL (HR=1.98, p=.007, Figure 1). Similarly, among pts who achieved a CR, median EFS for those with ALC ≥350/μL on day 28 was 42.1 mos compared to 13.9 mos in pts with ALC <350 cells/μL (HR=2.08, p=.006). Other factors associated with OS and EFS in univariable analyses were age at diagnosis, CG, WBC at diagnosis, and subsequent transplant (all p≤.04). In multivariable analysis the ALC on day 28 (<350 cells vs ≥350 cells/μL, p≤.0004 for OS and EFS) along with WBC at diagnosis (≤6.0 or >50.0 K/μL vs >6.0–50 K/μL, p≤.002 for OS and EFS) and CG (abnormal vs normal, p=.002 for OS and p=.02 for EFS) were independent prognostic factors of both outcomes. Combining these three factors by counting the number of poor features present yields a 3-group risk stratification: favorable (0–1 poor feature, 30% of pts, median OS and EFS not yet reached); intermediate (2 poor features, 45% of pts, estimated median OS and EFS 47.4 and 66.0 mos, respectively); unfavorable (3 poor features, 26% of pts, median OS and EFS 9.0 and 6.5 mos, respectively).
By identifying an additional prognostic marker—ALC—this study aims to optimize the current adult ALL treatment protocol by risk stratification. As an indicator of bone marrow recovery, ALC following induction therapy may demonstrate the body's immune surveillance against malignant cells. Further characterization of higher risk pts allows for modifications to therapeutic regimens, which may translate into improvements in long-term survival. In addition, this data suggests that targeting the immune system to improve ALC may be a worthwhile strategy in ALL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal