Abstract
Abstract 2541
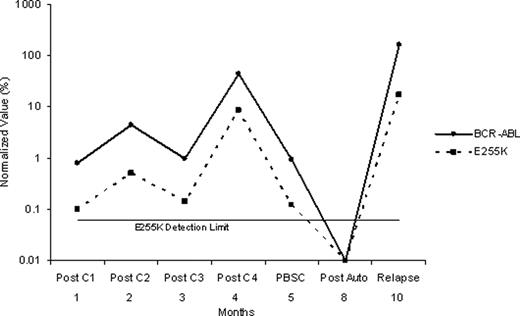
CALGB 10001 tested the feasibility of performing allogeneic or autologous stem cell transplantation (SCT) following combination chemotherapy and tyrosine kinase inhibition with imatinib in patients <60 years old with newly diagnosed Ph+ ALL. Patients received imatinib with each course of chemotherapy prior to SCT. A minimum of 12 months of maintenance therapy with imatinib was recommended following SCT or until minimal residual disease was no longer detected using quantitative real-time PCR (q-PCR). One goal of this study was to determine the incidence of ABL kinase domain mutations in patients who relapsed during or following treatment on CALGB 10001, and to evaluate the kinetics of the resistant clone. 58 patients (median age, 44 yrs) enrolled on CALGB 10001 between 11/2003 and 2/2010. After 3–4 courses of imatinib plus chemotherapy, 15 patients received TBI/etoposide and an alloSCT from a matched sibling donor; 9 (60%) remain in CR (median, 665+ days; range, 152+ to 1208+ days), 2 subsequently relapsed (days +236 and +354), and 4 had transplant-related mortality (TRM). 17 patients received TBI/etoposide/cyclophosphamide and autologous SCT; 10 (59%) remain in CR (median, 742+ days; range, 196+ to 1656+), 9 relapsed (range, 257–522 days), and none had TRM (updated from Wetzler et al, ASH 2007; abstract #2869). Thus, a total of 19 patients have relapsed (8 prior to SCT; 2 after alloSCT; 9 after autoSCT,) at a median of 5.9 months following SCT. All patients were monitored for BCR/ABL1 by q-PCR at sequential time points during and following treatment. Using direct sequencing methods (sensitivity 15–25%), an ABL kinase domain mutation was found in 12 of the 19 (63%) at relapse. The mutations included: 4 with Y253H, 3 T315I, 3 E255K, and 1 E255V. One patient had both a T315I and E255V mutation. All of these mutations are associated with imatinib resistance. To evaluate the kinetics of the resistant clone, we used a quantitative mutation-specific PCR assay (Gruber et al, Leukemia 19:2159, 2005). The sensitivity of the assay was shown to be 1% for T315I and E255V and 0.1% for Y253H and E255K. Using the q-PCR method, we found the resistant clone was detectable at diagnosis in 6 of the 12 patients with kinase domain mutations. In 4 patients, the resistant clone was not detectable until the time of relapse; however, for 3 of these cases the relapse occurred very early, within 2 months of starting treatment. In the remaining 2 cases, the resistant clone was first detected during treatment at 1 and 9 months prior to relapse (Figure 1). In most cases, the kinetics of the resistant clone mirrored the overall BCR/ABL1 population (Figure 2). Nevertheless, the resistant clone proportionally increased in size over time, and at relapse, the resistant clone became the dominant BCR-ABL1 clone. In summary, our series demonstrates that ABL kinase domain mutations occur commonly at relapse in Ph+ ALL despite aggressive therapy with imatinib plus combination chemotherapy followed by autoSCT and in some cases, alloSCT. The relapses appear to be due to the emergence of an imatinib-resistant subclone that is frequently present but subclinical at the time of diagnosis. Improved TKI-based therapies with activity against imatinib-resistant subclones instituted early during treatment of Ph+ ALL are needed to prevent emergence of clonal resistance and eventual relapse. Use of sensitive q-PCR assays to screen for these commonly pre-existing mutations at diagnosis, perhaps using a multiplex method, would provide useful clinical information to allow for optimal therapeutic management of Ph+ ALL.
Figure 1.
Figure 2.
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal