Abstract
Abstract 2515
The ETV6-RUNX1 (TEL-AML1) rearrangement comes from the translocation of two chromosomes t(12;21)(p12;q22), and represents one of the most frequently detected anomalies (15–30%) in the B-precursor Acute Lymphoblastic Leukemia (B-ALL). It must be identified by polymerase chain reaction (PCR) or fluorescent in situ hybridization (FISH) methods, since this translocation is not detected by conventional cytogenetic techniques. The prognostic value of ETV6-RUNX1 is still a matter of controversy. Recently, the group of the St Jude Children's Hospital reported an excellent outcome in patients carrying the translocation, whereas the BMF group did not find any significant difference in the survival of ETV6-RUNX1 positive patients when compared to ETV6-RUNX1 negative. The aim of our study has been to determine the prognostic impact of the ETV6-RUNX1 rearrangement in patients diagnosed of B-ALL in our Hospital, after a long period of follow-up and with the same Spanish treatment protocols (from PETHEMA and SHOP groups).
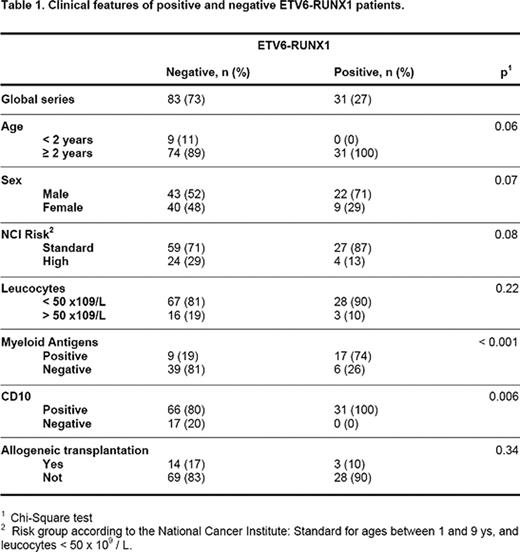
All patients with B-ALL diagnosis from January 1997 to May 2011 were included in the study: in total, 114 patients with a mean of age of 6 ys (0.3–14). The type of leukemia was ALL common (83 patients), pro-B (17 patients), pre-B (13 patients) and mature (1 patient). All the children over 1 yr received treatment according to PETHEMA group protocols, adjusted to the risk. Children under 1 yr were treated following SHOP group protocols. Seventeen patients received an allogeneic transplantation. The main clinical features of the positive and negative patients for ETV6-RUNX1 are detailed in table 1. ETV6-RUNX1 assay was performed in our laboratory by RT-PCR, according to the European BIOMED project methodology.
ETV6-RUNX1 was found in 31 of the 114 patients (27.2%). These patients showed a significantly higher frequency of myeloid antigens (p<0.001), and were always positive for CD10 (p=0.006). All cases of positive ETV6-RUNX1 were over 2 years old. No significant differences between positive and negative ETV6-RUNX1 were obtained when complete remissions (100 vs 80%), relapse (16 vs 20%) or deaths (10 vs 13%) were analyzed. Furthermore, estimation of disease free survival (DSF) at 14 ys for both groups were similar: 80 ± 8% for positive vs 66 ± 7% for negative (p=0.21, log-rank test). And the same happened for overall survival (OS): 87 ± 7% for positive vs 83 ± 5% for negative (p=0.4, log-rank test).
In our series, including patients with B-ALL treated with similar protocols with long periods of follow-up, we could not find differences between positive and negative ETV6-RUNX1 patients. It is well known that the intensity of the chemotherapy regimen and the age of inclusion in different protocols may influence the prognosis. Therefore, at present, it is still a matter of discussion if previous reported differences in the B-ALL ETV6-RUNX1 positive group could be explained by a different stratification in risk groups or by different chemotherapy regimens.
This work has been funded by a grant from AECC, Carmen Lavigne Prize 2010
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal