Abstract
Abstract 2375
The Notch signaling pathway is evolutionally conserved and has crucial roles in the control of fate decision and differentiation in numerous cell types. Although Notch1 is continuously expressed in differentiation myeloid cells, the role of Notch1 signaling in regulating differentiation remains controversial.
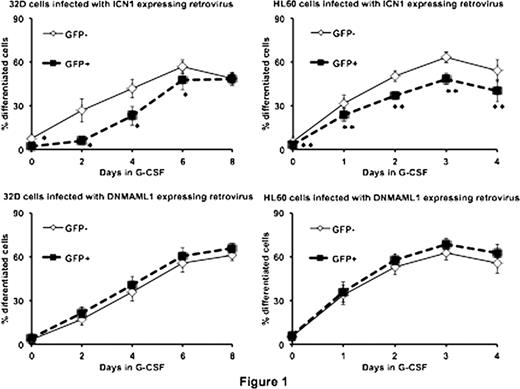
Here, we enhanced and/or suppressed Notch signaling in myeloblasts and then determined the effects of Notch signaling modulation on granulocytic differentiation. Specifically, we first transduced myeloblastic 32D and HL60 cells with retroviruses that express the intracellular domain of Notch1 (ICN1; pMSCV-ICN1-IRES-GFP) to activate Notch1 signaling, or alternatively expressed a dominant-negative form of Mastermind-like 1 (DNMAML1; pMSCV-DNMAML1-GFP) to inhibit Notch signaling. Then the transduced (GFP+) and untransduced (GFP-) cells were induced into granulocytic differentiation using G-CSF for 32D cells and ATRA for HL60 cells. The degree of granulocytic differentiation was then assessed by flow cytometric analysis of surface expression of CD11b, a marker of granulocytic lineage. We found that the percentage of differentiated cells (CD11bhigh for 32D and CD11b+ for HL60) in the ICN1 expressing (GFP+) group was significantly (p < 0.05) lower than that in the control (GFP-) group for most time-points that we examined, whereas the difference in the proportion of differentiated cells between DNMAML1 expressing (GFP+) and control (GFP-) groups was not statistically significant. These data suggest that forced activation of Notch1 signaling inhibits granulocytic differentiation, whereas endogenous Notch1 signaling appears not to have a major role in granulocytic maturation in these cell lines.
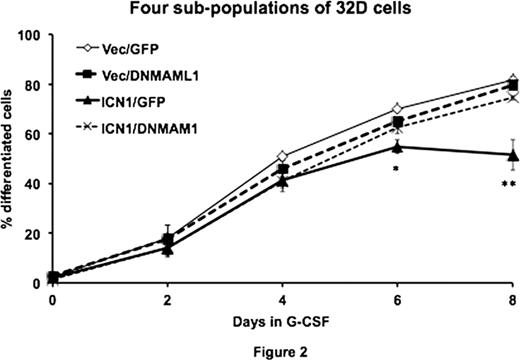
Next, We further studied the role of Notch1 signaling in granulopoiesis by first activating Notch1 signaling in 32D cells by stable expression of exogenous ICN1 followed by Notch inhibition via DNMAML1 expression within the same cells. The subsequent four sub-populations of 32D cells termed as Vec/GFP (control), Vec/DNMAML1 (cells with endogenous Notch signaling blocked by DNMAML1, ICN1/GFP (cells with activating Notch1), and ICN1/DNMAML1 (cells with activating Notch1 followed by Notch signaling inhibition) were then cultured with G-CSF and evaluated for differentiation by CD11b staining. We found that on days 6 and 8 after the induction of differentiation, the proportion of differentiated (CD11bhigh) cells in ICN1/GFP was significantly (p < 0.05) lower than those in the other sub-populations, supporting that ICN1 inhibits granulocytic differentiation of 32D cells. On the other hand, the difference in the proportion of differentiated cells between the other 3 sub-populations was not statistically significant at any time-points, suggesting that DNMAML1 reverses the phenotype induced by activated Notch1 and that endogenous Notch1 signaling may have no effect on granulocytic maturation. Real-time RT-PCR analysis of cytoplasmic expression of myeloperoxidase (MPO) indicated that the MPO expression reached maximal level by day 2 in control (Vec/GFP) cells, the peak was delayed until day 5 in ICN1/GFP cells. The peak expression was observed on day 3 in both Vec/DNMAML1 and ICN1/DNMAML1 cells, indicating that ICN1 represses or delays granulocytic differentiation and that DNMAML1 partially neutralizes such a phenotype. Furthermore, morphological analysis, viable cell count, and cell cycle analysis revealed that a subset of ICN1/GFP cells remained myeloblastic with proliferative capacity after the induction of granulocytic differentiation, supporting the idea that ICN1 inhibits granulocytic differentiation.
Griffin:Novartis Pharmaceuticals: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal