Abstract
Abstract 2272
Fresh whole blood (WB) collected from a “walking blood bank” is used by the U.S. military to supplement component therapy when blood component supplies are exhausted. Currently, WB is used for emergency transfusion within 24 hours of collection, before results of pathogen testing are available. A pathogen reduction technology (PRT), which uses riboflavin and ultraviolet light to damage nucleic acids in pathogens, is being considered as a transfusion-transmitted disease (TTD) risk mitigation measure. The effect of this technology on the hemostatic properties of whole blood, particularly on clotting capacity and clot lysis, are poorly understood, and optimal storage conditions are not defined. We previously reported that activated partial thrombin time (aPTT) and prothrombin time (PT) are prolonged by treatment; however, these tests do not always correlate with clinical findings. Thromboelastography is a more robust measure of clot formation and stability over time; we previously found that maximum amplitude (MA), which represents clot strength, did not decrease with PRT treatment and was preserved by storage at 4°C. Here we explore the effects of PRT on other parameters important to clotting capacity and clot lysis, and present the effects of WB storage at 4°C compared to 22°C.
WB treated with PRT demonstrates similar hemostatic function to non-treated WB, and storage at 4°C reduces degradation of blood components essential to clotting capacity and clot lysis compared to 22°C.
Under an IRB-approved protocol, 8 units per treatment group of WB were collected in CPD anticoagulant from healthy donors of normal hemostatic status according to standard blood donor guidelines. Pathogen reduction was performed using riboflavin and ultraviolet light (265–400nm phosphor; Mirasol® System, CaridianBCT) dosed at 80 J/mLRBC. Treatment groups included: control WB stored at 4° C (CON-04); control WB stored at 22° C (CON-22); PRT-treated WB stored at 4° C (PRT-04); and PRT-treated WB stored at 22° C (PRT-22). The hemostatic function of the blood was assessed at baseline, days 1–7, 10, 14, and 21. Factor VIII and fibrinogen were measured from assayed samples (BCS® XP system, Siemens). Thromboelastography (TEG®, Haemoscope Corp.) estimated total thrombin generation by calculating the first derivative of the TEG tracing, the Total Thrombus Generation variable (TTG). TEG was also used to measured lysis (LY30). Data were analyzed as repeated measures, followed by analysis of variance to assess interactions. Significance was set at p<0.05.
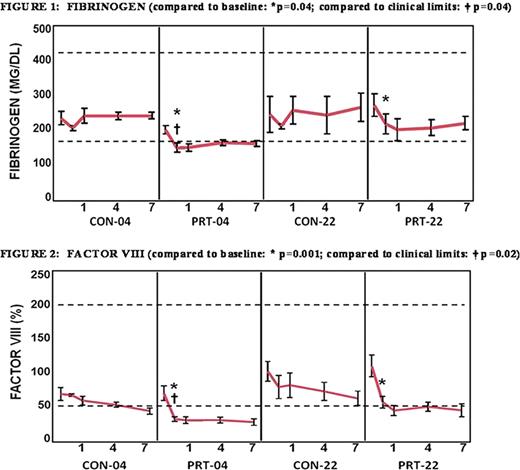
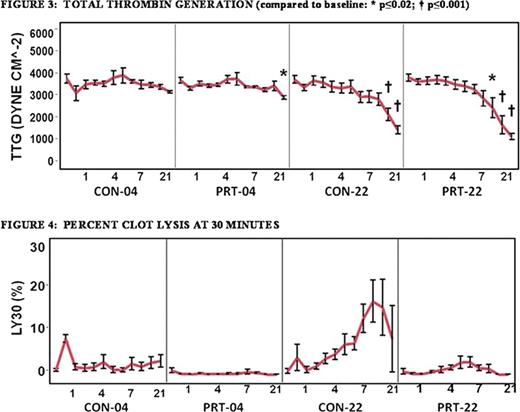
Treatment with PRT caused an initial drop in fibrinogen (baseline: 244 ± 77.5 mg/dL versus post-treatment: 185 ± 63.2 mg/dL, p≤0.04) and factor VIII (baseline: 96 ± 39% versus day post-treatment: 46 ± 23%, p≤0.001); however, levels stabilized thereafter (p≥0.987 and p≥0.871, respectively; see Fig. 1–2). Baseline fibrinogen levels were similar between groups p≥0.386). PRT-04 was the only group in which both fibrinogen and Factor VIII levels fell below clinical reference ranges (fibrinogen: p'0.039; factor VIII: p≤0.016). TTG was unaffected by PRT and was preserved through day 14 by storage at 4° C (p≥0.979, see Fig. 3), but only through day 10 when stored at 22°C (p≤0.290 at day 10). PRT treatment inhibited clot lysis (LY30) compared to storage at 22°C (p≤0.001), and variability was the lowest in the PRT-04 group (p≤0.001, see Fig. 4).
Our data demonstrate that pathogen reduction inhibited clot lysis. Decreased clot formation could conceivably account for the smaller degree of lysis; however, we previously found that MA is unaffected, and now demonstrated that thrombin generation was preserved despite a treatment-related decrease in factor VIII levels. While fibrinogen levels were diminished in the PRT-04 group, they were preserved in the PRT-22 group, which also demonstrated the diminished lysis. Cold storage preserved WB clotting capacity compared to storage at room temperature. The clinical significance of these findings has yet to be established; a coagulopathic animal hemorrhage model could determine whether the effects of PRT-induced lysis inhibition and cold storage are beneficial.
Mora:CaridianBCT: Research Funding. Pidcoke:CaridianBCT: Research Funding. Valdez-Delgado:CaridianBCT: Research Funding. Fedyk:CaridianBCT: Research Funding. Reddy:CaridianBCT: Employment, Research Funding. Goodrich:CaridianBCT: Employment, Research Funding. Cap:CaridianBCT: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal